User Tools
Sidebar
This is an old revision of the document!
Table of Contents
Drug Domain
The drug exposure domain concepts capture records about the utilization of a Drug when ingested or otherwise introduced into the body. A Drug is a biochemical substance formulated in such a way that when administered to a Person it will exert a certain physiological or biochemical effect. The following products are not considered Drugs, but Devices:
- Radiopharmaceuticals
- Contrast material for imaging
- Nutritional products and supplements, including baby formula. In reality that results in the slightly arbitrary and in some cases difficult to ascertain situation that solutions of salts for parental use are Drugs (hydrating patients and maintaining ionic balance), while the addition of nutrients such as glucose or vitamins makes them devices (feeding patients).
- Products directly derived from blood (e.g. erythocytes or plasma)
Drug domain Concepts should be used in the drug_concept_id of the DRUG_EXPOSURE the DRUG_ERA and the DOSE_ERA (both only at the Ingredient level) tables, or in the value_as_concept_id field of the OBSERVATION or MEASUREMENT table (e.g. for Measurements like “Plasma level”).
Overall Structure
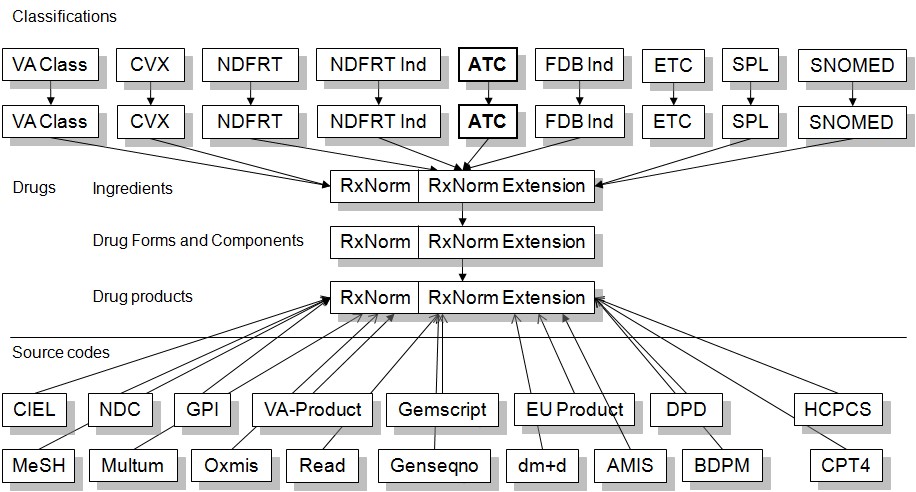
The Drug Domain consists of Source Concepts (below the horizontal line), Drugs (Standard Concepts) and Classifications (Classification Concepts). As usual, Source Concepts are non-Standard Concepts that are used as coding schemes in various databases. As such, they are mapped to Standard Concepts.
Standard Concepts are based on the RxNorm and RxNorm Extension vocabularies. All Standard RxNorm and RxNorm Extension Concepts are used in the drug_concept_id fields.
RxNorm is the foundation for the combined Domain space. All valid RxNorm Concepts (invalid_reason is null) are used as Standard Concepts. However, RxNorm only contains Drug Concepts for products sold in the United States. All Concepts derived from international products are availabe in the RxNorm Extension vocabulary. Apart from the different vocabulary_id, both RxNorm and RxNorm Extension form a combined Concept space with identical rules for relationships and Concept Ancestry.
RxNorm and RxNorm Extension Concepts are hierarchical. Therefore the Concepts can also be used as Class Concepts: descendants of any Standard Concept in the CONCEPT_ANCESTOR table can expected to be a correct semantic match in a query. For example, a query for the descendants of an ingredient should turn up all drug products (Clinical Drug or Branded Drug) containing that ingredient.
On top of this combined RxNorm/RxNorm Extension Drug Domain are drug classification systems. These are derived from a variety of different vocabularies. Note: Any classification Concept may exist in different classifications, and therefore cannot be considered as unique. For example, NSAID Analgesics exist with very similar memberships as ETC, VA Class and SNOMED classes.
Concept Classes
Those for the Source and Classification Concepts are specific to the source vocabulary (see there). The Concept Classes for the Drug Concepts are based on a subset of RxNorm Term Types:
| Term Type | Name | Concept Class |
|---|---|---|
| IN | Ingredient | Ingredient |
| PIN | Precise Ingredient | Ingredient (standard_concept = NULL) |
| MIN | Multiple Ingredients | Not implemented |
| BN | Brand Name | Brand Name |
| SCDC | Semantic Clinical Drug Component | Clinical Drug Component |
| SCDF | Semantic Clinical Drug Form | Clinical Drug Form |
| SCDG | Semantic Clinical Dose Form Group | Not implemented |
| SCD | Semantic Clinical Drug | Clinical Drug or Quantified Clinical Drug |
| SBDC | Semantic Branded Drug Component | Branded Drug Component |
| SBDF | Semantic Branded Drug Form | Branded Drug Form |
| SBDG | Semantic Branded Dose Form Group | Not implemented |
| SBD | Semantic Branded Drug | Branded Drug or Quantified Branded Drug |
| GPCK | Generic Pack | Clinical Pack |
| BPCK | Brand Name Pack | Branded Pack |
| DF | Dose Form | Dose Form |
| DFG | Dose Form Group | Not implemented |
| PSN | Prescribable Name | Not implemented |
| SY | Synonym | Implemented in the CONCEPT_SYNONYM table |
| TMSY | Tall Man Lettering Synonym | Not implemented |
| ET | Dose Form Entry Term | Not implemented |
Concepts of the Concept Classses “Clinical Drug” and “Branded Drug” (SCD and SBD) of liquid formuations have drug strength provided as concentrations. These often exist in pairs – with the total quantity provided or not. RxNorm does represent this distinction using different Term Types, however, we defined addtional Concept Classes “Quantified Clincal Drug” and “Quantified Branded Drug”.
In addition to the RxNorm-derived Concept Classes, international coding schemes brake drugs down according to their package or box size. This is due to the fact that prescription drugs are available as prepackaged products, while most prescription drugs in the US get packaged at the time of the prescription filling. Note, that these boxes are different from the RxNorm packs. Packs represent the smallest unit of drug product combinations, while boxes define the total amount of units sold as a product. In addition to boxes, international coding scheme also distinguish products of different manufacturers or resellers (suppliers). To make those coding schemes work seamlessly with the RxNorm system, additional Concept Classes were defined in the RxNorm Extension vocabulary:
| Vocab | Name | Ingre dient | Drug strength | Dose form | Brand name | Quantity factor | Box size | Pack content | Supplier |
|---|---|---|---|---|---|---|---|---|---|
| RxNorm | Ingredient | x | |||||||
| Brand Name | x | ||||||||
| Clinical Drug Component | x | x | |||||||
| Clinical Drug Form | x | x | |||||||
| Clinical Drug | x | x | x | ||||||
| Branded Drug Component | x | x | x | ||||||
| Branded Drug Form | x | x | x | ||||||
| Branded Drug | x | x | x | x | |||||
| Clinical Pack | x | x | |||||||
| Branded Pack | x | x | x | ||||||
| Rxnorm (not explicit) | Quantified Clinical Drug | x | x | x | x | ||||
| Quantified Branded Drug | x | x | x | x | x | ||||
| RxNorm Extension | Clinical Drug Box | x | x | x | x | ||||
| Branded Drug Box | x | x | x | x | x | ||||
| Quantified Clinical Box | x | x | x | x | x | ||||
| Quantified Branded Box | x | x | x | x | x | x | |||
| Clinical Pack Box | x | x | x | x | |||||
| Branded Pack Box | x | x | x | x | x | ||||
| Marketed Product | x | x | x | optional | optional | optional | optional | x |
The Concept Classes of RxNorm are equivalent to
Relationships
All Source Vocabularies are mapped to the RxNorm Standard Concepts, and Multilex and DMD also to the DMD Standard Concepts. The mapping is either constructed from the vocabulary source and in some cases curated by the vocabulary team.
NDC, GPI, Multum and Genseqno to RxNorm mapping
All these are provided by RxNorm.
CVX, OXMIS, Read and CPT4 to RxNorm mapping
These are manually curated by the vocabulary team
MeSH to RxNorm mapping
This is provided by the UMLS.
CIEL, VA Product, Gemscript and HCPCS to RxNorm mapping
These are inferred from public source and curated by the vocabulary team.
DMD, Gemscript and Multilex to RxNorm/DMD mapping
These are inferred from public source and curated by the vocabulary team.
Internal relationships
All relationships provided by RxNorm are loaded as is, but renamed to follow the relationship_id formatting rules and allow for bi-directional mappings, see RxNorm. All internal relationships of DMD are derived from the source and converted to generate “pseudo”-RxNorm relationship, omitting the Drug Form and Drug Component Concepts. See DMD for details. All hierarchical relationships of the drug classes are loaded for the source as is and used to form the CONCEPT_ANCESTOR table.
