User Tools
Sidebar
This is an old revision of the document!
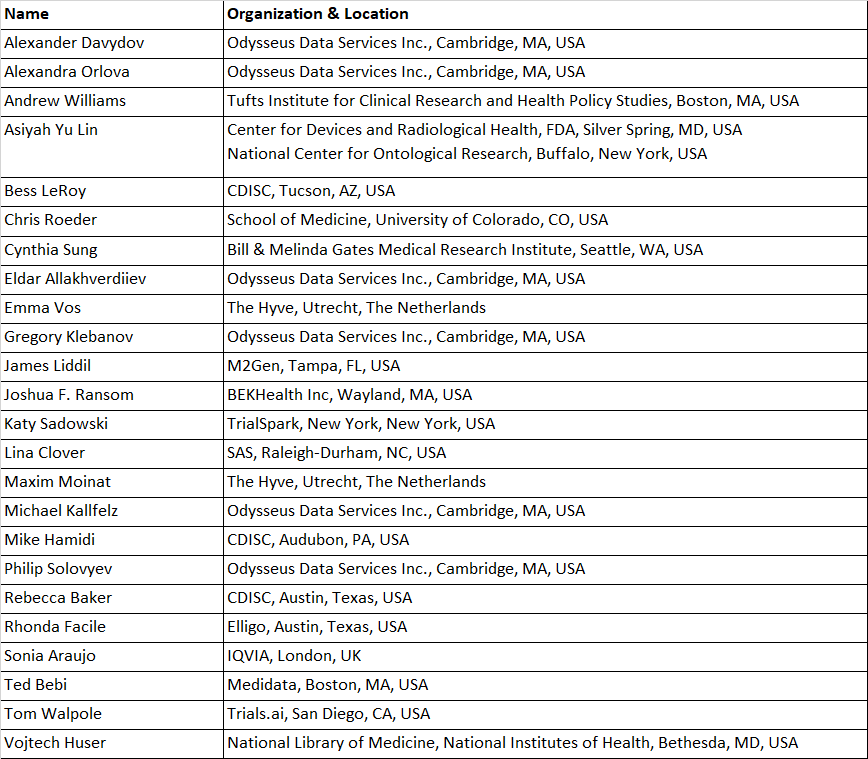
Clinical Trials Working Group
Objective: To allow adequate representation of clinical trial data in OMOP.
First use case: To convert clinical trial data in CDISC SDTM format to OMOP, with a view to allowing trial planning optimization.
Approach: We advocate minimum changes to the OMOP CDM and Standardized Vocabularies because we want to ensure minimum impact on OHDSI tools like Atlas, whilst providing a value-add SDTM-to-OMOP conversion with minimum data loss. We have proposed conventions introducing new concepts and modifiers, but no new CDM tables; and providing guidance for ETL developers where appropriate. Our proposals are built on OMOP CDM v6 and the Oncology extension, with v5.3.1 backward compatibility.
Status:
- Proposals submitted to the OHDSI community in July 2020.
- Currently applying our proposed conventions to the PHUSE database (a synthetic SDTM database).
WG Lead: Sonia Araujo, IQVIA, London, UK (sonia.araujo@iqvia.com)
Important Links
Conventions proposal submitted to OHDSI
https://github.com/OHDSI/CommonDataModel/issues/358
Group GitHub repository
https://github.com/OHDSI/ClinicalTrialsWGETL
Meetings
Schedule: Biweekly calls on Fridays, 3pm UK time (10am Eastern)
Recordings
| Q4 2020 | Q3 2020 | Q2 2020 | Q1 2020 |
|---|---|---|---|
| 02 Oct | 18 Sep | 26 Jun | 20 Mar |
| 04 Sep | 22 May | 06 Mar (partial) | |
| 21 Aug | 15 May | 07 Feb | |
| 14 Aug | 01 May | 10 Jan | |
| 03 Apr |
Q4 2019 06 Dec - initial presentations from the Hyve, Medidata and Odysseus
Want to contribute to the group? Email sonia.araujo@iqvia.com