User Tools
Sidebar
This is an old revision of the document!
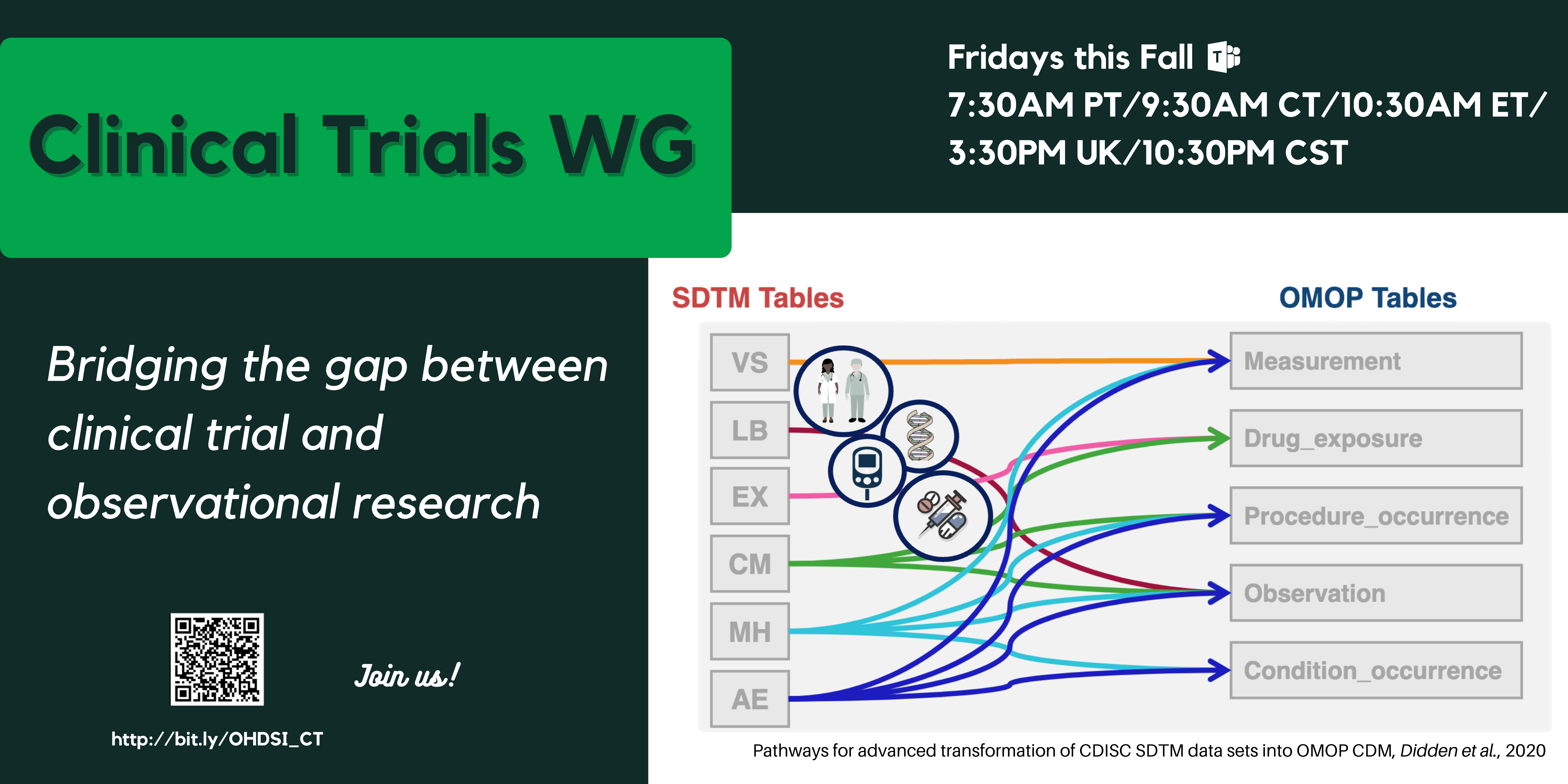
Clinical Trials Working Group
Objective: To allow adequate representation of clinical trial data in OMOP.
First use case: To convert clinical trial data in CDISC SDTM format to OMOP, with a view to allowing trial planning optimization.
Approach: We advocate minimum changes to the OMOP CDM and Standardized Vocabularies because we want to ensure minimum impact on OHDSI tools like Atlas, whilst providing a value-add SDTM-to-OMOP conversion with minimum data loss. We have proposed conventions introducing new concepts and modifiers, but no new CDM tables; and providing guidance for ETL developers where appropriate. Our proposals are built on OMOP CDM v6 and the Oncology extension, with v5.3.1 backward compatibility.
Status:
- Currently applying our proposed conventions to the PHUSE database (a synthetic SDTM database).
- Proposals submitted to the OHDSI community in July 2020.
Important Links
Conventions proposal submitted to OHDSI
https://github.com/OHDSI/CommonDataModel/issues/358
Group GitHub repository
https://github.com/OHDSI/ClinicalTrialsWGETL
Recent WG Presentation Slides
Meetings
Schedule: Biweekly calls on Fridays, 7:30AM PT/9:30AM CT/10:30AM ET/3:30PM UK time on Teams here
Note on accessing Teams channel above:
* If you use the Teams desktop app and have multiple accounts, ensure you are already in the OHDSI Teams account before clicking the link above
* Alternatively, select the “web app” button in the link above for accessing meetings on your browser
Membership
WG co-leads: Mike Hamidi, Pfizer, US (Kamiar.Hamidi@pfizer.com) and Zhen Lin, Robot Bacon, US (Zhen.Lin@ohdsi.org)
Active members
Other members
| Name | Organization | Location | |
|---|---|---|---|
| Eldar Allakhverdiiev | Odysseus Data Services Inc. | Cambridge, MA, USA | |
| Sonia Araujo * | IQVIA | London, UK | |
| Ted Bebi | Medidata | Boston, MA, USA | |
| Lina Clover | SAS | Raleigh-Durham, NC, USA | |
| Alexander Davydov | Odysseus Data Services Inc. | Cambridge, MA, USA | |
| Shawn Dolley | Open Global Health | Arlington, Virginia, USA | |
| Rhonda Facile | CDISC | Austin, Texas, USA | |
| Vojtech Huser | National Library of Medicine, National Institutes of Health | Bethesda, MD, USA | |
| Gregory Klebanov | Odysseus Data Services Inc. | Cambridge, MA, USA | |
| Bess LeRoy | CDISC | Tucson, AZ, USA | |
| James Liddil | M2Gen | Tampa, FL, USA | |
| Maxim Moinat | The Hyve | Utrecht, The Netherlands | |
| Alexandra Orlova | Odysseus Data Services Inc. | Cambridge, MA, USA | |
| Joshua F. Ransom | BEKHealth Inc | Wayland, MA, USA | |
| Chris Roeder | School of Medicine, University of Colorado | CO, USA | |
| Cynthia Sung | Bill & Melinda Gates Medical Research Institute | Seattle, WA, USA | |
| Emma Vos | The Hyve | Utrecht, The Netherlands | |
| Andrew Williams | Tufts Institute for Clinical Research and Health Policy Studies | Boston, MA, USA |
* Past leads
Want to contribute to the group? Opt-in here.