User Tools
Sidebar
Clinical Trials Working Group
Objective: To allow adequate representation of clinical trial data in OMOP.
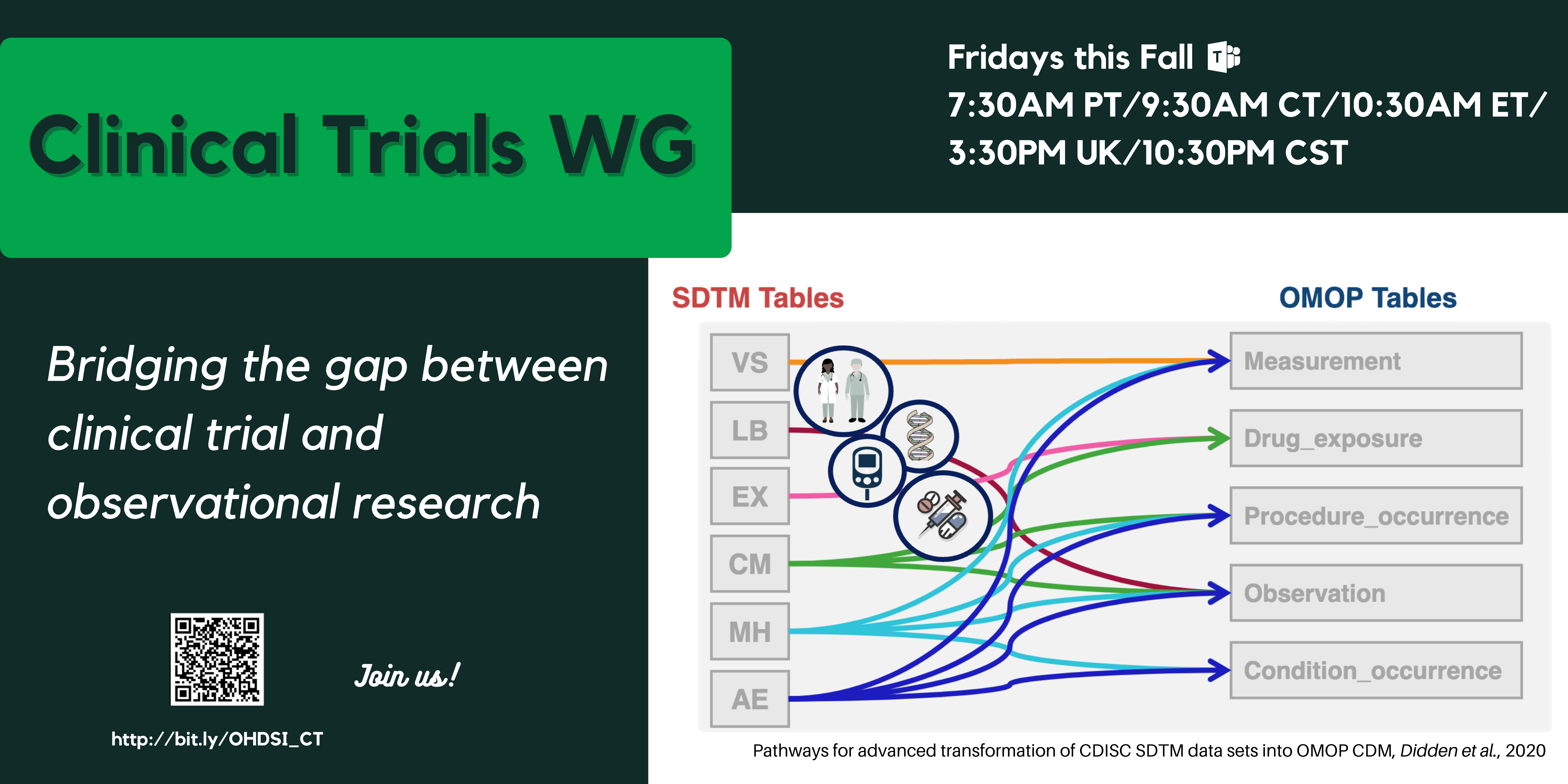
First use case: To convert clinical trial data in CDISC SDTM format to OMOP, with a view to allowing trial planning optimization.
Approach: We advocate minimum changes to the OMOP CDM and Standardized Vocabularies because we want to ensure minimum impact on OHDSI tools like Atlas, whilst providing a value-add SDTM-to-OMOP conversion with minimum data loss. We have proposed conventions introducing new concepts and modifiers, but no new CDM tables; and providing guidance for ETL developers where appropriate. Our proposals were originally built on OMOP CDM v6 and the Oncology extension, with v5.3 backward compatibility. In a new v5.4 the additions from the Oncology extension became standard, which made our changes minimal, thereby, making our proposals fully compatible with v5.4.
Status:
- 2022: CTWG was given access to 20 Vivli clinical study packages in the SDTM format. The CTWG team is doing an inventory of those study packages in order to prioritize SDTM-to-OMOP mappings. The existing CTWG guidance topics will be further assessed and new ones identified where necessary.
- 2021: CTWG did an assessment of clinical trial data providers where SDTM data could be accessed. This eventually led to discussions with Vivli (i.e., general data usage agreements and platform feasibility evaluation).
- 2020: Used a synthetic representation of the CDISC SDTM data via PHUSE Test Data. Initial guidance topics were codified but require further testing with diverse real world SDTM data. The CTWG proposals submitted to the OHDSI community in July 2020.
Important Links
Conventions proposal submitted to OHDSI
https://github.com/OHDSI/CommonDataModel/issues/358
Group GitHub repository
https://github.com/OHDSI/ClinicalTrialsWGETL
Recent WG Updates: Nov. 2022 Presentation; Oct. 2021 Presentation and Slides
Meetings
Bi-weekly Friday WG Meeting Schedule is accessible on the OHDSI Teams environment.
* Note on accessing Teams channel:
- If you use the Teams desktop app and have multiple accounts, ensure you are already in the OHDSI Teams account before clicking the link above
- Alternatively, select the “web app” button in the link above for accessing meetings on your browser
- Join our WG here if you haven't: https://ohdsi.org/join-the-journey/.
Membership
WG co-leads: Mike Hamidi, and TBD
Active members
Other members
* Past leads
Want to contribute to the group? Opt-in here.
Click here to catch up recent calls and job openings