OMOP Common Data Model V5.1
Consolidated Documentation
License
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
© 2014 Observational Health Data Sciences and Informatics
This work is based on work by the Observational Medical Outcomes Partnership (OMOP) and used under license from the FNIH at http://omop.fnih.org/publiclicense.
All derivative work after the OMOP CDM v4 specification is dedicated to the public domain. Observational Health Data Sciences and Informatics (OHDSI) has waived all copyright and related or neighboring rights to the extent allowed by law.
Background
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The Observational Medical Outcomes Partnership (OMOP) was a public-private partnership established to inform the appropriate use of observational healthcare databases for studying the effects of medical products. Over the course of the 5-year project and through its community of researchers from industry, government, and academia, OMOP successfully achieved its aims to:
- Conduct methodological research to empirically evaluate the performance of various analytical methods on their ability to identify true associations and avoid false findings,
- Develop tools and capabilities for transforming, characterizing, and analyzing disparate data sources across the health care delivery spectrum, and
- Establish a shared resource so that the broader research community can collaboratively advance the science.
The results of OMOP's research has been widely published and presented at scientific conferences, including annual symposia.
The OMOP Legacy continues…
The community is actively using the OMOP Common Data Model for their various research purposes. Those tools will continue to be maintained and supported, and information about this work is available in the public domain.
The Observational Health Data Sciences and Informatics (OHDSI) has been established as a multi-stakeholder, interdisciplinary collaborative to create open-source solutions that bring out the value of observational health data through large-scale analytics. The OHDSI collaborative includes all of the original OMOP research investigators, and will develop its tools using the OMOP Common Data Model. Learn more at ohdsi.org.
The OMOP Common Data Model will continue to be an open-source, community standard for observational healthcare data. The model specifications and associated work products will be placed in the public domain, and the entire research community is encouraged to use these tools to support everybody's own research activities.
The Role of the Common Data Model
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
No single observational data source provides a comprehensive view of the clinical data a patient accumulates while receiving healthcare, and therefore none can be sufficient to meet all expected outcome analysis needs. This explains the need for assessing and analyzing multiple data sources concurrently using a common data standard. This standard is provided by the OMOP Common Data Model (CDM).
The CDM is designed to support the conduct of research to identify and evaluate associations between interventions (drug exposure, procedures, healthcare policy changes etc.) and outcomes caused by these interventions (condition occurrences, procedures, drug exposure etc.). Outcomes can be efficacious (benefit) or adverse (safety risk). Often times, specific patient cohorts (e.g., those taking a certain drug or suffering from a certain disease) may be defined for treatments or outcomes, using clinical events (diagnoses, observations, procedures, etc.) that occur in predefined temporal relationships to each other. The CDM, combined with its standardized content (via the Standardized Vocabularies), will ensure that research methods can be systematically applied to produce meaningfully comparable and reproducible results.
Design Principles
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The CDM is designed to include all observational health data elements (experiences of the patient receiving health care) that are relevant for analysis use cases to support the generation of reliable scientific evidence about disease natural history, healthcare delivery, effects of medical interventions, the identification of demographic information, health care interventions and outcomes.
Therefore, the CDM is designed to store observational data to allow for research, under the following principles:
- Suitability for purpose. The CDM aims at providing data organized in a way optimal for analysis, rather than for the purpose of operational needs of health care providers or payers.
- Data protection. All data that might jeopardize the identity and protection of patients, such as names, precise birthdays etc. are limited. Exceptions are possible where the research expressly requires more detailed information, such as precise birth dates for the study of infants.
- Design of domains. The domains are modeled in a person-centric relational data model, where for each record the identity of the person and a date is captured as a minimum.
- Rationale for domains. Domains are identified and separately defined in an Entity-relationship model if they have an analysis use case and the domain has specific attributes that are not otherwise applicable. All other data can be preserved as an observation in an entity-attribute-value structure.
- Standardized Vocabularies. To standardize the content of those records, the CDM relies on the Standardized Vocabularies containing all necessary and appropriate corresponding standard healthcare concepts.
- Reuse of existing vocabularies. If possible, these concepts are leveraged from national or industry standardization or vocabulary definition organizations or initiatives, such as the National Library of Medicine, the Department of Veterans' Affairs, the Center of Disease Control and Prevention, etc.
- Maintaining source codes. Even though all codes are mapped to the Standardized Vocabularies, the model also stores the original source code to ensure no information is lost.
- Technology neutrality. The CDM does not require a specific technology. It can be realized in any relational database, such as Oracle, SQL Server etc., or as SAS analytical datasets.
- Scalability. The CDM is optimized for data processing and computational analysis to accommodate data sources that vary in size, including databases with up to hundreds of millions of persons and billions of clinical observations.
- Backwards compatibility. All changes from previous CDMs are clearly delineated. Older versions of the CDM can be easily created from this CDMv5, and no information is lost that was present previously.
Details of the Model
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The CDM defines table structures in a person-centric way. At a minimum, the tables have a foreign key into the Person table and a date. This allows for a longitudinal view on all the healthcare-relevant events. The exceptions from this rule are the standardized health system data tables, which are linked directly to events of the various domains.
To represent the relevant domains, the CDM contains the following 39 tables:
| Table name | Description |
|---|---|
| Standardized Vocabularies | |
| CONCEPT | The CONCEPT table contains records that uniquely identify each fundamental unit of meaning used to express clinical information. Concepts are derived from source vocabularies, which represent clinical information across different domains (e.g. conditions, drugs, procedures) through the use of source codes and associated descriptions. Some concepts are designated as standard concepts, meaning these concepts can be used within the OMOP Common Data Model and within standardized analytics. Each standard concept has a primary domain, which defines the location where the concept would be expected to be observed within OMOP Common Data Model. |
| VOCABULARY | The VOCABULARY table includes a list of the Vocabularies collected from various sources or created de-novo by the OMOP community. This reference table is populated with a single record for each vocabulary source and includes a descriptive name and other associated attributes for the vocabulary. |
| DOMAIN | The DOMAIN table includes a list of OMOP-defined Domains the Concepts of the Standardized Vocabularies can belong to. A domain defines the set of allowable concepts for each standardized field. This reference table is populated with a single record for each domain and includes a descriptive name for the Domain. |
| CONCEPT_CLASS | The CONCEPT_CLASS table includes a list of the classifications used to differentiate concepts within a given vocabulary. Concept Classes are defined by the source Vocabulary, but might be slightly altered to fit OMOP CDM table constraints. This reference table is populated with a single record with a descriptive name for each Concept Class. |
| CONCEPT_RELATIONSHIP | The CONCEPT_RELATIONSHIP table contains records that define direct relationships between any two concepts and the nature of the relationship. The type of relationship is defined in the Relationship table. |
| RELATIONSHIP | The RELATIONSHIP table provides a reference list of all allowable types of relationships that can be used to associate any two concepts in the CONCEPT_RELATIONSHIP table. |
| CONCEPT_SYNONYM | The CONCEPT_SYNONYM table is used to store alternate names and descriptions for a concept. |
| CONCEPT_ANCESTOR | The CONCEPT_ANCESTOR table contains records that define the inferred hierarchical relationships between all Standard Concepts. The concept ancestor table is fully derived from the CONCEPT, CONCEPT_RELATIONSHIP, and RELATIONSHIP tables and is designed to simplify observational analysis by providing the complete hierarchical relationships between Concepts. |
| SOURCE_TO_CONCEPT_MAP | The SOURCE_TO_CONCEPT_MAP table is a legacy data structure within the OMOP Common Data Model, recommended for use in extract, transform, and load (ETL) processes to maintain local source codes which are not available as concepts in the Standardized Vocabularies. |
| DRUG_STRENGTH | The DRUG_STRENGTH table contains structured content about the amount or concentration and associated units of a specific ingredient within a particular drug product. The drug strength table is a supplemental file to support standardized analysis of drug utilization. |
| COHORT_DEFINITION | The COHORT_DEFINITION table contains records to define each derived Cohort through an associated description and syntax and can store operational programming code to instantiate the cohort within a OMOP common data model. |
| ATTRIBUTE_DEFINITION | The ATTRIBUTE_DEFINITION table contains records to define each attribute through an associated description and syntax. Attributes are derived elements that can be selected or calculated for a subject within a cohort. |
| Standardized meta-data | |
| CDM_SOURCE | The CDM_SOURCE table contains detail about the source database and the process used to transform the data into the OMOP common data model. If a source database is derived from multiple data feeds, the integration of those disparate sources is expected to be documented in the ETL specifications. |
| Standardized clinical data | |
| PERSON | The PERSON table contains records that uniquely identify each patient in the source data who has time at-risk to have clinical events recorded within the source systems. |
| OBSERVATION_PERIOD | The OBSERVATION_PERIOD table contains records which uniquely define the spans of time for which a Person is at-risk to have clinical events recorded within the source systems, even if no events in fact are recorded (healthy patient with no healthcare interactions). |
| SPECIMEN | The SPECIMEN table contains the records identifying each biological sample from a person. |
| DEATH | The DEATH table contains the clinical event for how and when a Person dies. A person can have up to one record if the source system contains evidence about the Death. |
| VISIT_OCCURRENCE | The VISIT_OCCURRENCE table contains the spans of time a person continuously receives medical services from one or more providers at a facility in a given setting within the health care system. Visits are classified into 4 settings: outpatient care, inpatient confinement, emergency room, and long-term care. Persons may transition between these settings over the course of an episode of care. |
| PROCEDURE_OCCURRENCE | The PROCEDURE_OCCURRENCE table contains records of activities or processes ordered by, or carried out by, a healthcare provider on the patient to have a diagnostic or therapeutic purpose. |
| DRUG_EXPOSURE | The DRUG_EXPOSURE table captures records about the inferred utilization of a biochemical substance with a physiological therapeutic effect when ingested or otherwise introduced into the body. Drugs include prescription and over-the-counter medicines, vaccines, and large-molecule biologic therapies. |
| DEVICE_EXPOSURE | The device exposure domain captures information about a person’s exposure to a foreign physical object or instrument that which is used for diagnostic or therapeutic purposes through a mechanism beyond chemical action. Devices include implantable objects (e.g. pacemakers, stents, artificial joints), durable medical equipment and supplies (e.g. bandages, crutches, syringes), and other instruments used in medical procedures (e.g. sutures, defibrillators). |
| CONDITION_OCCURRENCE | Conditions are records of a Person suggesting the presence of a disease or medical condition stated as a diagnosis, a sign or a symptom, which is either observed by a Provider or reported by the patient. |
| MEASUREMENT | The MEASUREMENT table contains records of Measurement, i.e. structured values (numerical or categorical) obtained through systematic and standardized examination or testing of a Person or Person's sample. The MEASUREMENT table contains both orders and results of such Measurements as laboratory tests, vital signs, quantitative findings from pathology reports, etc |
| NOTE | The NOTE table captures unstructured information that was recorded by a provider or a patient in free text notes on a given date. |
| OBSERVATION | The OBSERVATION table captures clinical facts about a Person obtained in the context of examination, questioning or a procedure. Any data that cannot be represented by any other domains, such as social and lifestyle facts, medical history, family history, etc. are recorded here. |
| FACT_RELATIONSHIP | The FACT_RELATIONSHIP table contains records about the relationships between facts stored as records in any table of the CDM. Relationships can be defined between facts from the same domain (table), or different domains. Examples of Fact Relationships include: Person relationships (parent-child), care site relationships (hierarchical organizational structure of facilities within a health system), indication relationship (between drug exposures and associated conditions), usage relationships (of devices during the course of an associated procedure), or facts derived from one another (measurements derived from an associated specimen). |
| Standardized health system data | |
| LOCATION | |
| CARE_SITE | The CARE_SITE table contains a list of uniquely identified institutional (physical or organizational) units where healthcare delivery is practiced (offices, wards, hospitals, clinics, etc.). |
| PROVIDER | The PROVIDER table contains a list of uniquely identified health care providers. These are individuals providing hands-on healthcare to patients, such as physicians, nurses, midwives, physical therapists etc. |
| Standardized health economics | |
| PAYER_PLAN_PERIOD | The PAYER_PLAN_PERIOD table captures the unique combination of the period of time that a Person is continuously enrolled under a specific health Plan benefit structure from a given Payer as well as covered family members. |
| VISIT_COST | The VISIT_COST table captures the costs of health visit of a patient which are not itemized to specific procedures, drugs, or devices used within the Visit. |
| PROCEDURE_COST | The PROCEDURE_COST table captures the cost of a Procedure performed on a Person. The information about the cost is only derived from the amount of money paid for the Procedure. |
| DRUG_COST | The DRUG_COST table captures records indicating the cost of a Drug Exposure. The information about the cost is defined by the amount of money paid by the person and payer for the drug, as well as the charged cost of the drug. |
| DEVICE_COST | The DEVICE_COST table captures the cost of a medical device or supply used on a Person. The information about the cost is only derived from the amount of money paid for the device. |
| Standardized derived elements | |
| COHORT | The COHORT table contains records derived as a set of subjects that satisfy a given set of inclusion criteria for a duration of time COHORT_DEFINITION table. Cohorts can be constructed of patients (Persons), Providers or Visits. |
| COHORT_ATTRIBUTE | The COHORT_ATTRIBUTE table contains attributes associated with each subject within a cohort, as defined by a given set of criteria for a duration of time. The definition of the Cohort Attribute is contained in the ATTRIBUTE_DEFINITION table. |
| DRUG_ERA | A Drug Era is defined as a span of time when the Person is assumed to be exposed to a particular active ingredient, i.e. successive periods of Drug Exposures combined under certain rules to produce continuous Drug Eras. |
| DOSE_ERA | A Dose Era is defined as a span of time when the Person is assumed to be exposed to a constant dose of a specific active ingredient. |
| CONDITION_ERA | A Condition Era is defined as a span of time when the Person is assumed to have a given condition. |
Data Model Conventions
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
There are a number of implicit and explicit conventions that have been adopted in the CDM. Developers of methods that run methods against the CDM need to understand these conventions.
General conventions of data tables
The CDM is platform-independent. Data types are defined generically using ANSI SQL data types (VARCHAR, INTEGER, FLOAT, DATE, TIME, CLOB). Precision is provided only for VARCHAR. It reflects the minimal required string length and can be expanded within a CDM instantiation. The CDM does not prescribe the date and time format. Standard queries against CDM may vary for local instantiations and date/time configurations.
In most cases, the first field in each table ends in “_id”, containing a record identifier that can be used as a foreign key in another table.
General conventions of fields
Variable names across all tables follow one convention:
| Notation | Description |
|---|---|
| <entity>_SOURCE_VALUE | Verbatim information from the source data, typically used in ETL to map to CONCEPT_ID, and not to be used by any standard analytics. For example, condition_source_value = ‘787.02’ was the ICD-9 code captured as a diagnosis from the administrative claim |
| <entity>_ID | Unique identifiers for key entities, which can serve as foreign keys to establish relationships across entities For example, person_id uniquely identifies each individual. visit_occurrence_id uniquely identifies a PERSON encounter at a point of care. |
| <entity>_CONCEPT_ID | Foreign key into the Standardized Vocabularies (i.e. the standard_concept attribute for the corresponding term is true), which serves as the primary basis for all standardized analytics For example, condition_concept_id = 31967 contains reference value for SNOMED concept of ‘Nausea’ |
| <entity>_SOURCE_CONCEPT_ID | Foreign key into the Standardized Vocabularies representing the concept and terminology used in the source data, when applicable For example, condition_source_concept_id = 35708202 denotes the concept of ‘Nausea’ in the MedDRA terminology; the analogous condition_concept_id might be 31967, since SNOMED-CT is the Standardized Vocabularies for most clinical diagnoses and findings. |
| <entity>_TYPE_CONCEPT_ID | Delineates the origin of the source information, standardized within the Standardized Vocabularies For example, drug_type_concept_id can allow analysts to discriminate between ‘Pharmacy dispensing’ and ‘Prescription written’ |
Representation of content through Concepts
In CDM data tables the meaning of the content of each record is represented using Concepts. Concepts are stored with their concept_id as foreign keys to the CONCEPT table in the Standardized Vocabularies, which contains Concepts necessary to describe the healthcare experience of a patient. If a Standard Concept does not exist or cannot be identified, the Concept with the concept_id 0 is used, representing a non-existing or unmappable concept.
Records in the CONCEPT table contain all the detailed information about it (name, relationships, types etc.). Concepts, Concept Relationships and other information relating to Concepts contained in the tables of the Standardized Vocabularies..
Difference between Concept IDs and Source Values
Many tables contain equivalent information multiple times: As a Source Value, a Source Concept and as a Standard Concept.
- Source Values contains the codes from public code systems such as ICD-9-CM, NDC, CPT-4 etc. or local controlled vocabularies (such as F for female and M for male) copied from the source data. Source Values are stored in the _source_value field in the data tables.
- Concepts are CDM-specific entities that represent the meaning of a clinical fact. Most concepts are based on code systems used in healthcare (called Source Concepts), while others were created de-novo (concept_code = “OMOP generated”). Concepts have unique IDs across all domains.
- Source Concepts are the concepts that represent the code used in the source. Source Concepts are only used for common healthcare code systems, but not for OMOP-generated Concepts. Source Concepts are stored in the source_concept_id field in the data tables.
- Standard Concepts are those concepts that are used to define the unique meaning of a clinical entity. For each entity there is one Standard Concept. Standard Concepts are typically drawn from existing public vocabulary sources. Concepts that have the equivalent meaning to a Standard Concept are mapped to the Standard Concept. Standard Concepts are referred to in the concept_id field of the data tables.
Source Values are only provided for convenience and quality assurance (QA) purposes. Source Values and Source Concepts are optional, while Standard Concepts are mandatory. Source Values may contain information that is only meaningful in the context of a specific data source.
Difference between general Concepts and Type Concepts
Type Concepts (ending in _type_concept_id) and general Concepts (ending in _concept_id) are part of many tables. The former are special Concepts with the purpose of indicating where the data are derived from in the source. For example, the Type Concept field can be used to distinguish a DRUG_EXPOSURE record that is derived from a pharmacy-dispensing claim from one indicative of a prescription written in an electronic health record (EHR).
Time span of available data
Data tables for clinical data contain a date stamp (ending in _date, _start_date or _end_date), indicating when that clinical event occurred. As a rule, no record can be outside of a valid OBSERVATION_PERIOD time period. Clinical information that relates to events happened prior the first OBSERVATION_PERIOD, it will be captured as a record in the OBSERVATION table of 'Medical history' (concept_id = 43054928), with the observation_date set to the first observation_period_start_date of that patient, and the value_as_concept_id set to the corresponding concept_id for the condition/drug/procedure that occurred in the past. No data occurring after the last observation_period_end_date can be valid records in the CDM.
Content of each table
For the tables of the main domains of the CDM it is imperative that used concepts are strictly limited to the domain. For example, the CONDITION_OCCURRENCE table contains only information about conditions (diagnoses, signs, symptoms), but no information about procedures. Not all source coding schemes adhere to such rules. For example, ICD-9-CM codes, which contain mostly diagnoses of human disease, also contain information about the status of patients having received a procedure: V25.5 “Encounter for insertion of implantable subdermal contraceptive” defines a procedure and is therefore stored in the PROCEDURE_OCCURRENCE table.
Differentiating between source values, source concept ids, and standard concept ids
Each table contains fields for source values, source concept ids, and standard concept ids.
- Source values are fields to maintain the verbatim information from the source database, are stored as unstructured text, and are generally not to be used by any standardized analytics.
- Source concept ids provide a repeatable representation of the source concept, when the source data are drawn from a commonly-used internationally-recognized vocabulary that has been distributed with the OMOP Common Data Model. Specific use cases where source vocabulary-specific analytics are required can be accommodated by the use of the source concept id fields, but these are generally not applicable across disparate data sources. The standard concept id fields are strongly suggested to be used in all standardized analytics, as specific vocabularies have been established within each data domain to facilitate standardization of both structure and content within the OMOP Common Data Model.
The following provide conventions for processing source data using these three fields in each domain:
When processing data where the source value is either free text or a reference to a coding scheme that is not contained within the Standardized Vocabularies:
- Map all source values directly to standard concept_ids. Store these mappings in the SOURCE_TO_CONCEPT_MAP table.
- If the source code is not mappable to a vocabulary term, the source_concept_id field is set to 0
When processing your data where source value is a reference to a coding scheme contained within the Standardized Vocabularies:
- Map all your source values to the corresponding concept_ids in the source vocabulary. Store the result in the source_concept_id field.
- If the source code follows the same formatting as the distributed vocabulary, the mapping can be directly obtained from the CONCEPT table using the CONCEPT_CODE field.
- If the source code uses alternative formatting (ex. format has removed decimal point from ICD-9 codes), you will need to perform the formatting transformation within the ETL. In this case, you may wish to store the mappings from original codes to source concept ids in the SOURCE_TO_CONCEPT_MAP table.
- If the source code is not mappable to a vocabulary term, the source_concept_id field is set to 0
- Use the CONCEPT_RELATIONSHIP table to identify the standard concept_id that corresponds to the source_concept_id in the domain.
- Each source_concept_id can have 1 or more Standard concept_id mapped to it. Each Standard concept_id belongs to only one primary domain, but when a source concept_id maps to multiple standard concept_ids, it is possible for that source_concept_id to result in records being produced across multiple domains. For example, HCPCS code for infusion of a drug will map to a concept in the procedure domain of the infusion and a different concept in the drug domain for the product infused. It is also possible for one source_concept_id to map to multiple standard concept_ids within the same domain. For example, ICD-9 for ‘viral hepatitis with hepatic coma’ maps to SNOMED ‘viral hepatitis’ and a different concept for ‘hepatic coma’ in which case multiple condition_occurrence records will be generated for the one source value record.
- If the source_concept_id is not mappable to any standard concept_id, the concept_id field is set to 0.
- Write the data record into table(s) corresponding to the domain of the standard concept_id(s).
- If the source value is mapped to source_concept_id, but the source_concept_id is not mapped to a standard concept_id, then the domain for the data record, and hence it's table location, is determined by the domain_id field of the CONCEPT record the source_concept_id refers to. The standard concept_id is set to 0.
- If the source value cannot be mapped to a source_concept_id or standard concept_id, then direct the data record to the most appropriate CDM domain based on your local knowledge of the intent of the source data and associated value. For example, if the unmappable source_value came from a ‘diagnosis’ table, then in the absence of other information, you may choose to record that fact in the CONDITION_OCCURRENCE table.
Each standard concept_id field has a set of allowable concept_id values. The allowable values are defined by the domain of the concepts. For example, there is a domain concept of ‘Gender’, for which there are only two allowable standard concepts of practical use (8507- ‘Male’, 8532- ‘Female’) and one allowable generic concept to represent a standard notion of ‘no information’ (concept_id = 0).
There is no constraint on allowed concept_ids within the source_concept_id fields.
Custom source_to_concept_maps
When the source data uses coding systems that are not currently in the Standardized Vocabularies (e.g. ICPC codes for diagnoses), the convention is to store the mapping of such source codes to Standard Concepts in the SOURCE_TO_CONCEPT_MAP table. The codes used in the data source can be recorded in the source_value fields, but no source_concept_id will be available.
Custom source codes are not allowed to map to Standard Concepts that are marked as invalid.
Changes from CDM Version 4
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The changes can be summarized into the following categories:
- New tables and fields
- Changes to existing tables and fields
- Changes in the conventions of the content of tables and fields
- Changes to the vocabulary
New tables and fields
A number of new tables where introduced to reflect the additional information that will be stored in the CDM.
Glossary of Terms
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
| Term | Abbr. | Description |
|---|---|---|
| Ancestor | The higher level Concept in a hierarchical relationship. Note that ancestors and descendants can be many levels apart from each other. | |
| Average Wholesale Price | AWP | The price manufacturers set for prescription drugs to be purchased at the wholesale level to pharmacies and healthcare provider. |
| Centers for Disease Control and Prevention | CDC | The Centers for Disease Control and Prevention is a United States federal agency under the Department of Health and Human Services. It works to protect public health and safety by providing information to enhance health decisions. |
| Common Data Model | CDM | The CDM intends to facilitate observational analyses of disparate healthcare databases. The CDM defines table structures for each of the data entities (e.g., Persons, Visit Occurrence, Drug Exposure, Condition Occurrence, Observation, Procedure Occurrence, etc.). It includes observational data elements that are relevant to identifying exposure to various treatments and defining condition occurrence. The CDM includes both the Standardized Vocabularies of terms and the entity domain tables. |
| Concept | A concept is the basic unit of information. Concepts may be grouped into a given domain. A concept is a unique term that has a unique and static identifier/name, belongs to a domain, and may exist in relation to other concepts. The vertical relationships consist of “is a” statements that form a logical hierarchy. In general, concepts above a given concept are referred to as ancestors and those below as descendants. | |
| Conceptual Data Model | A conceptual data model is a map of concepts and their relationships. This describes the semantics of an organization and represents a series of assertions about its nature. Specifically, it describes the things of significance to an organization (entity classes), about which it is inclined to collect information, and characteristics of (attributes) and associations between pairs of those things of significance (relationships). | |
| Data mapping | It is the data element mappings between two distinct data models, terminologies, or concepts. Data mapping is the process of creating data element mappings between two distinct data models. Data mapping is used as a first step for a wide variety of data integration tasks. | |
| Demographics | Demographics refer to selected characteristics of persons. Demographics may include data such as race, age, sex, date of birth, location, etc. | |
| Descendant | The lower level Concept in a hierarchical relationship. Note that ancestors and descendants can be many levels apart from each other. | |
| Design Principle | An organized arrangement of one or more elements or principles for a purpose. It identifies core principles and best practices to assist developers to produce software. Thoroughly understanding the goals of stakeholders and designing systems with those goals in mind are the best approaches to successfully deliver results. | |
| Electronic Health Record | EHR | Electronic health record refers to an individual person's medical record in digital format. It may be made up of electronic medical records from many locations and/or sources. The EHR is a longitudinal electronic record of person health information generated by one or more encounters in any care delivery setting. Included in this information are person demographics, progress notes, problems, medications, vital signs, past medical history, immunizations, laboratory data and radiology reports. |
| Electronic Medical Record | EMR | An electronic medical record is a computerized medical record created in an organization that delivers care, such as a hospital or outpatient setting. Electronic medical records tend to be a part of a local stand-alone health information system that allows storage, retrieval and manipulation of records. This document will reference EHR moving forward even if specific data source might internally use EMR definition. |
| Extract Transform Load | ETL | Process of getting data out of one data store (Extract), modifying it (Transform), and inserting it into a different data store (Load). |
| Health Insurance Portability and Accountability Act | HIPAA | A federal law that was designed to allow portability of health insurance between jobs. In addition, it required the creation of a federal law to protect personally identifiable health information; if that did not occur by a specific date (which it did not), HIPAA directed the Department of Health and Human Services (DHHS) to issue federal regulations with the same purpose. DHHS has issued HIPAA privacy regulations (the HIPAA Privacy Rule) as well as other regulations under HIPAA. |
| Logical Data Model | Logical data models are graphical representation of the business requirements. They describe the things of importance to an organization and how they relate to one another, as well as business definitions and examples. The logical data model can be validated and approved by a business representative, and can be the basis of physical database design. | |
| Primary Care Provider | PCP | A health care provider designated as responsible to provide general medical care to a patient, including evaluation and treatment as well as referral to specialists. |
| Protected Health Information | PHI | Protected health information under HIPAA includes any individually identifiable health information. Identifiable refers not only to data that is explicitly linked to a particular individual (that's identified information). It also includes health information with data items which reasonably could be expected to allow individual identification. De-identified information is that from which all potentially identifying information has been removed. |
| Terminology | Technical or special terms used in a business or special subject area. | |
| Vocabulary | A computerized list (as of items of data or words) used for reference (as for information retrieval or word processing). |
Standardized Vocabularies
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
These tables contain detailed information about the Concepts used in all of the CDM fact tables. The content of the Standardized Vocabularies tables is not generated anew by each CDM implementation. Instead, it is maintained centrally as a service to the community.
A number of assumptions were made for the design of the Standardized Vocabularies tables:
- There is one design which will accommodate all different source terminologies and classifications.
- All terminologies are loaded into the CONCEPT table.
- The key is a newly created concept_id, not the original code of the terminology, because source codes are not unique identifiers across terminologies.
- Some Concepts are declared Standard Concepts, i.e. they are used to represent a certain clinical entity in the data. All Concepts may be Source Concepts; they represent how the entity was coded in the source. Standard Concepts are identified through the standard_concept field in the CONCEPT table.
- Records in the CONCEPT_RELATIONSHIP table define semantic relationships between Concepts. Such relationships can be hierarchical or lateral.
- Records in the CONCEPT_RELATIONSHIP table are used to map Source codes to Standard Concepts, replacing the mechanism of the SOURCE_TO_CONCEPT_MAP table used in prior Standardized Vocabularies versions. The SOURCE_TO_CONCEPT_MAP table is retained as an optional aid to bookkeeping codes not found in the Standardized Vocabularies.
- Chains of hierarchical relationships are recorded in the CONCEPT_ANCESTOR table. Ancestry relationships are only recorded between Standard Concepts that are valid (not deprecated) and are connected through valid and hierarchical relationships in the RELATIONSHIP table (flag defines_ancestry).
The advantage of this approach lies in the preservation of codes and relationships between them without adherence to the multiple different source data structures, a simple design for standardized access, and the optimization of performance for analysis. Navigation among Standard Concepts does not require knowledge of the source vocabulary. Finally, the approach is scalable and future vocabularies can be integrated easily. On the other hand, extensive transformation of source data to the Vocabulary is required and not every source data structure and original source hierarchy can be retained.
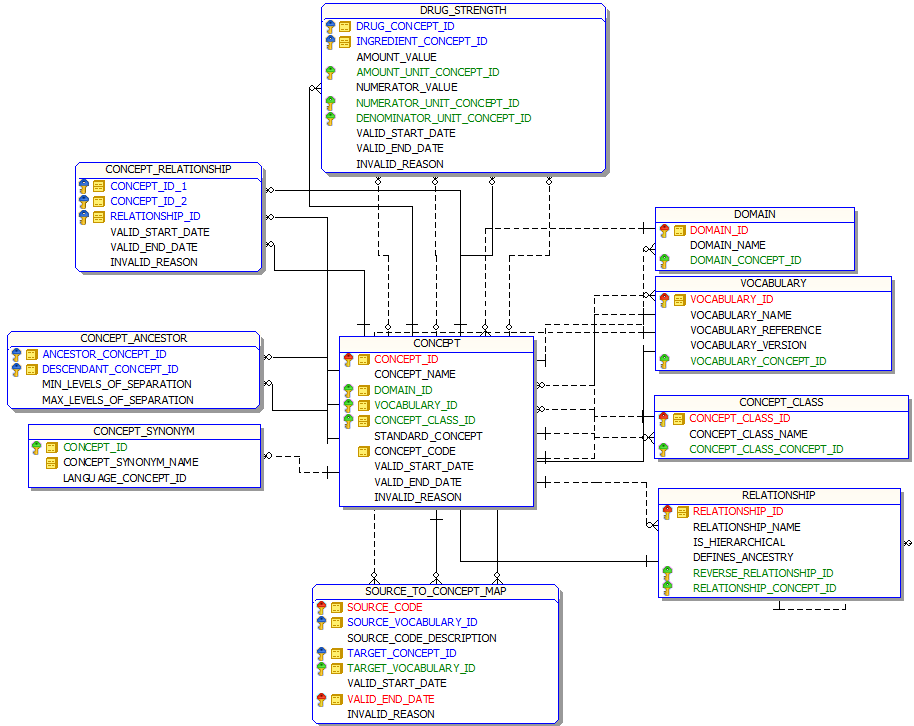
Below is an entity-relationship diagram highlighting the tables within the Vocabulary portion of the OMOP Common Data Model:
CONCEPT table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The Standardized Vocabularies contains records, or Concepts, that uniquely identify each fundamental unit of meaning used to express clinical information in all domain tables of the CDM. Concepts are derived from vocabularies, which represent clinical information across a domain (e.g. conditions, drugs, procedures) through the use of codes and associated descriptions. Some Concepts are designated Standard Concepts, meaning these Concepts can be used as normative expressions of a clinical entity within the OMOP Common Data Model and within standardized analytics. Each Standard Concept belongs to one domain, which defines the location where the Concept would be expected to occur within data tables of the CDM.
Concepts can represent broad categories (like “Cardiovascular disease”), detailed clinical elements (”Myocardial infarction of the anterolateral wall”) or modifying characteristics and attributes that define Concepts at various levels of detail (severity of a disease, associated morphology, etc.).
Records in the Standardized Vocabularies tables are derived from national or international vocabularies such as SNOMED-CT, RxNorm, and LOINC, or custom Concepts defined to cover various aspects of observational data analysis. For a detailed description of these vocabularies, their use in the OMOP CDM and their relationships to each other please refer to the Specifications.
| Field | Required | Type | Description |
|---|---|---|---|
| concept_id | Yes | integer | A unique identifier for each Concept across all domains. |
| concept_name | Yes | varchar(255) | An unambiguous, meaningful and descriptive name for the Concept. |
| domain_id | Yes | varchar(20) | A foreign key to the DOMAIN table the Concept belongs to. |
| vocabulary_id | Yes | varchar(20) | A foreign key to the VOCABULARY table indicating from which source the Concept has been adapted. |
| concept_class_id | Yes | varchar(20) | The attribute or concept class of the Concept. Examples are “Clinical Drug”, “Ingredient”, “Clinical Finding” etc. |
| standard_concept | No | varchar(1) | This flag determines where a Concept is a Standard Concept, i.e. is used in the data, a Classification Concept, or a non-standard Source Concept. The allowables values are 'S' (Standard Concept) and 'C' (Classification Concept), otherwise the content is NULL. |
| concept_code | Yes | varchar(50) | The concept code represents the identifier of the Concept in the source vocabulary, such as SNOMED-CT concept IDs, RxNorm RXCUIs etc. Note that concept codes are not unique across vocabularies. |
| valid_start_date | Yes | date | The date when the Concept was first recorded. The default value is 1-Jan-1970, meaning, the Concept has no (known) date of inception. |
| valid_end_date | Yes | date | The date when the Concept became invalid because it was deleted or superseded (updated) by a new concept. The default value is 31-Dec-2099, meaning, the Concept is valid until it becomes deprecated. |
| invalid_reason | No | varchar(1) | Reason the Concept was invalidated. Possible values are D (deleted), U (replaced with an update) or NULL when valid_end_date has the default value. |
Conventions
Concepts in the Common Data Model are derived from a number of public or proprietary terminologies such as SNOMED-CT and RxNorm, or custom generated to standardize aspects of observational data. Both types of Concepts are integrated based on the following rules:
- All Concepts are maintained centrally by the CDM and Vocabularies Working Group. Additional concepts can be added, as needed, upon request.
- For all Concepts, whether they are custom generated or adopted from published terminologies, a unique numeric identifier concept_id is assigned and used as the key to link all observational data to the corresponding Concept reference data.
- The concept_id of a Concept is persistent, i.e. stays the same for the same Concept between releases of the Standardized Vocabularies.
- A descriptive name for each Concept is stored as the Concept Name as part of the CONCEPT table. Additional names and descriptions for the Concept are stored as Synonyms in the CONCEPT_SYNONYM table.
- Each Concept is assigned to a Domain. For Standard Concepts, these is always a single Domain. Source Concepts can be composite or coordinated entities, and therefore can belong to more than one Domain. The domain_id field of the record contains the abbreviation of the Domain, or Domain combination. Please refer to the Standardized Vocabularies Specification for details of the Domain Assignment.
- For details of the Vocabularies adopted for use in the OMOP CDM refer to the Standardized Vocabularies Specification.
- Concept Class designation are attributes of Concepts. Each Vocabulary has its own set of permissible Concept Classes, although the same Concept Class can be used by more than one Vocabulary. Depending on the Vocabulary, the Concept Class may categorize Concepts vertically (parallel) or horizontally (hierarchically). See the specification of each Vocabulary for details.
- Concept Class attributes should not be confused with Classification Concepts. These are separate Concepts that have a hierarchical relationship to Standard Concepts or each other, while Concept Classes are unique Vocabulary-specific attributes for each Concept.
- For Concepts inherited from published terminologies, the source code is retained in the concept_code field and can be used to reference the source vocabulary.
- Standard Concepts (designated as 'S' in the standard_concept field) may appear in CDM tables in all *_concept_id fields, whereas Classification Concepts ('C') should not appear in the CDM data, but participate in the construction of the CONCEPT_ANCESTOR table and can be used to identify Descendants that may appear in the data. See CONCEPT_ANCESTOR table. Non-standard Concepts can only appear in *_source_concept_id fields and are not used in CONCEPT_ANCESTOR table. Please refer to the Standardized Vocabularies Specifications for details of the Standard Concept designation.
- All logical data elements associated with the various CDM tables (usually in the <domain>_type_concept_id field) are called Type Concepts, including defining characteristics, qualifying attributes etc. They are also stored as Concepts in the CONCEPT table. Since they are generated by OMOP, their is no meaningful concept_code.
- The lifespan of a Concept is recorded through its valid_start_date, valid_end_date and the invalid_reason fields. This allows Concepts to correctly reflect at which point in time were defined. Usually, Concepts get deprecatd if their meaning was deamed ambigous, a duplication of another Conncept, or needed revision for scientific reason. For example, drug ingredients get updated when different salt or isomer variants enter the market. Usually, drugs taken off the market do not cause a deprecation by the terminology vendor. Since observational data are valid with respect to the time they are recorded, it is key for the Standardized Vocabularies to provide even obsolete codes and maintain their relationships to other current Concepts .
- Concepts without a known instantiated date are assigned valid_start_date of ‘1-Jan-1970’.
- Concepts that are not invalid are assigned valid_end_date of ‘31-Dec-2099’.
- Deprecated Concepts (with a valid_end_date before the release date of the Standardized Vocabularies) will have a value of 'D' (deprecated without successor) or 'U' (updated). The updated Concepts have a record in the CONCEPT_RELATIONSHIP table indicating their active replacement Concept.
- Values for concept_ids generated as part of Standardized Vocabularies will be reserved from 0 to 2,000,000,000. Above this range, concept_ids are available for local use and are guaranteed not to clash with future releases of the Standardized Vocabularies.
VOCABULARY table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The VOCABULARY table includes a list of the Vocabularies collected from various sources or created de novo by the OMOP community. This reference table is populated with a single record for each Vocabulary source and includes a descriptive name and other associated attributes for the Vocabulary.
| Field | Required | Type | Description |
|---|---|---|---|
| vocabulary_id | Yes | varchar(20) | A unique identifier for each Vocabulary, such as ICD9CM, SNOMED, Visit. |
| vocabulary_name | Yes | varchar(255) | The name describing the vocabulary, for example “International Classification of Diseases, Ninth Revision, Clinical Modification, Volume 1 and 2 (NCHS)” etc. |
| vocabulary_reference | Yes | varchar(255) | External reference to documentation or available download of the about the vocabulary. |
| vocabulary_version | Yes | varchar(255) | Version of the Vocabulary as indicated in the source. |
| vocabulary_concept_id | Yes | integer | A foreign key that refers to a standard concept identifier in the CONCEPT table for the Vocabulary the VOCABULARY record belongs to. |
Conventions
- There is one record for each Vocabulary. One Vocabulary source or vendor can issue several Vocabularies, each of them creating their own record in the VOCABULARY table. However, the choice of whether a Vocabulary contains Concepts of different Concept Classes, or when these different classes constitute separate Vocabularies cannot precisely be decided based on the definition of what constitutes a Vocabulary. For example, the ICD-9 Volume 1 and 2 codes (ICD9CM, containing predominantly conditions and some procedures and observations) and the ICD-9 Volume 3 codes (ICD9Proc, containing predominantly procedures) are realized as two different Vocabularies. On the other hand, SNOMED-CT codes of the class Condition and those of the class Procedure are part of one and the same Vocabulary. Please refer to the Standardized Vocabularies Specifications for details of each Vocabulary.
- The vocabulary_id field contains an alphanumerical identifier, that can also be used as the abbreviation of the Vocabulary name.
- The record with vocabulary_id = 'None' is reserved to contain information regarding the current version of the Entire Standardized Vocabularies.
- The vocabulary_name field contains the full official name of the Vocabulary, as well as the source or vendor in parenthesis.
- Each Vocabulary has an entry in the CONCEPT table, which is recorded in the vocabulary_concept_id field. This is for purposes of creating a closed Information Model, where all entities in the OMOP CDM are covered by a unique Concept.
- In past versions of the VOCABULARY table, the vocabulary_id used to be a numerical value. A conversion table between these old and new IDs is given below:
| vocabulary_id previously | vocabulary_id Version 5 |
|---|---|
| 0 | None |
| 1 | SNOMED |
| 2 | ICD9CM |
| 3 | ICD9Proc |
| 4 | CPT4 |
| 5 | HCPCS |
| 6 | LOINC |
| 7 | NDFRT |
| 8 | RxNorm |
| 9 | NDC |
| 10 | GPI |
| 11 | UCUM |
| 12 | Gender |
| 13 | Race |
| 14 | Place of Service |
| 15 | MedDRA |
| 16 | Multum |
| 17 | Read |
| 18 | OXMIS |
| 19 | Indication |
| 20 | ETC |
| 21 | ATC |
| 22 | Multilex |
| 24 | Visit |
| 28 | VA Product |
| 31 | SMQ |
| 32 | VA Class |
| 33 | Cohort |
| 34 | ICD10 |
| 35 | ICD10PCS |
| 36 | Drug Type |
| 37 | Condition Type |
| 38 | Procedure Type |
| 39 | Observation Type |
| 40 | DRG |
| 41 | MDC |
| 42 | APC |
| 43 | Revenue Code |
| 44 | Ethnicity |
| 45 | Death Type |
| 46 | Mesh |
| 47 | NUCC |
| 48 | Specialty |
| 49 | LOINC |
| 50 | SPL |
| 53 | Genseqno |
| 54 | CCS |
| 55 | OPCS4 |
| 56 | Gemscript |
| 57 | HES Specialty |
| 58 | Note Type |
| 59 | Domain |
| 60 | PCORNet |
| 61 | Obs Period Type |
| 62 | Visit Type |
| 63 | Device Type |
| 64 | Meas Type |
| 65 | Currency |
| 66 | Relationship |
| 67 | Vocabulary |
| 68 | Concept Class |
| 69 | Cohort Type |
| 70 | ICD10CM |
| 71 | ICDO3 |
DOMAIN table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The DOMAIN table includes a list of OMOP-defined Domains the Concepts of the Standardized Vocabularies can belong to. A Domain defines the set of allowable Concepts for the standardized fields in the CDM tables. For example, the “Condition” Domain contains Concepts that describe a condition of a patient, and these Concepts can only be stored in the condition_concept_id field of the CONDITION_OCCURRENCE and CONDITION_ERA tables. This reference table is populated with a single record for each Domain and includes a descriptive name for the Domain.
| Field | Required | Type | Description |
|---|---|---|---|
| domain_id | Yes | varchar(20) | A unique key for each domain. |
| domain_name | Yes | varchar(255) | The name describing the Domain, e.g. “Condition”, “Procedure”, “Measurement” etc. |
| domain_concept_id | Yes | integer | A foreign key that refers to an identifier in the CONCEPT table for the unique Domain Concept the Domain record belongs to. |
Conventions
- There is one record for each Domain. The domains are defined by the tables and fields in the OMOP CDM that can contain Concepts describing all the various aspects of the healthcare experience of a patient.
- The domain_id field contains an alphanumerical identifier, that can also be used as the abbreviation of the Domain.
- The domain_name field contains the unabbreviated names of the Domain.
- Each Domain also has an entry in the Concept table, which is recorded in the domain_concept_id field. This is for purposes of creating a closed Information Model, where all entities in the OMOP CDM are covered by unique Concept.
- Past versions of the OMOP CDM did not support the notion of a Domain.
CONCEPT_CLASS table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The CONCEPT_CLASS table is a reference table, which includes a list of the classifications used to differentiate Concepts within a given Vocabulary. This reference table is populated with a single record for each Concept Class:
| Field | Required | Type | Description |
|---|---|---|---|
| concept_class_id | Yes | varchar(20) | A unique key for each class. |
| concept_class_name | Yes | varchar(255) | The name describing the Concept Class, e.g. “Clinical Finding”, “Ingredient”, etc. |
| concept_class_concept_id | Yes | integer | A foreign key that refers to an identifier in the CONCEPT table for the unique Concept Class the record belongs to. |
Conventions
- There is one record for each Concept Class. Concept Classes are used to create additional structure to the Concepts within each Vocabulary. Some Concept Classes are unique to a Vocabulary (for example “Clinical Finding” in SNOMED), but others can be used across different Vocabularies. The separation of Concepts through Concept Classes can be semantically horizontal (each Class subsumes Concepts of the same hierarchical level, akin to sub-Vocabularies within a Vocabulary) or vertical (each Class subsumes Concepts of a certain kind, going across hierarchical levels). For example, Concept Classes in SNOMED are vertical: The classes “Procedure” and “Clinical Finding” define very granular to very generic Concepts. On the other hand, “Clinical Drug” and “Ingredient” Concept Classes define horizontal layers or strata in the RxNorm vocabulary, which all belong to the same concept of a Drug.
- The concept_class_id field contains an alphanumerical identifier, that can also be used as the abbreviation of the Concept Class.
- The concept_class_name field contains the unabbreviated names of the Concept Class.
- Each Concept Class also has an entry in the Concept table, which is recorded in the concept_class_concept_id field. This is for purposes of creating a closed Information Model, where all entities in the OMOP CDM are covered by unique Concepts.
- Past versions of the OMOP CDM did not have a separate reference table for all Concept Classes. Also, the content of the old concept_class and the new concept_class_id fields are not always identical. A conversion talbe can be found here:
| concept_class previously | concept_class_id Version 5 |
|---|---|
| Administrative concept | Admin Concept |
| Admitting Source | Admitting Source |
| Anatomical Therapeutic Chemical Classification | ATC |
| Anatomical Therapeutic Chemical Classification | ATC |
| APC | Procedure |
| Attribute | Attribute |
| Biobank Flag | Biobank Flag |
| Biological function | Biological Function |
| Body structure | Body Structure |
| Brand Name | Brand Name |
| Branded Drug | Branded Drug |
| Branded Drug Component | Branded Drug Comp |
| Branded Drug Form | Branded Drug Form |
| Branded Pack | Branded Pack |
| CCS_DIAGNOSIS | Condition |
| CCS_PROCEDURES | Procedure |
| Chart Availability | Chart Availability |
| Chemical Structure | Chemical Structure |
| Clinical Drug | Clinical Drug |
| Clinical Drug Component | Clinical Drug Comp |
| Clinical Drug Form | Clinical Drug Form |
| Clinical finding | Clinical Finding |
| Clinical Pack | Clinical Pack |
| Concept Relationship | Concept Relationship |
| Condition Occurrence Type | Condition Occur Type |
| Context-dependent category | Context-dependent |
| CPT-4 | Procedure |
| Currency | Currency |
| Death Type | Death Type |
| Device Type | Device Type |
| Discharge Disposition | Discharge Dispo |
| Discharge Status | Discharge Status |
| Domain | Domain |
| Dose Form | Dose Form |
| DRG | Diagnostic Category |
| Drug Exposure Type | Drug Exposure Type |
| Drug Interaction | Drug Interaction |
| Encounter Type | Encounter Type |
| Enhanced Therapeutic Classification | ETC |
| Enrollment Basis | Enrollment Basis |
| Environment or geographical location | Location |
| Ethnicity | Ethnicity |
| Event | Event |
| Gender | Gender |
| HCPCS | Procedure |
| Health Care Provider Specialty | Provider Specialty |
| HES specialty | Provider Specialty |
| High Level Group Term | HLGT |
| High Level Term | HLT |
| Hispanic | Hispanic |
| ICD-9-Procedure | Procedure |
| Indication or Contra-indication | Ind / CI |
| Ingredient | Ingredient |
| LOINC Code | Measurement |
| LOINC Multidimensional Classification | Meas Class |
| Lowest Level Term | LLT |
| MDC | Diagnostic Category |
| Measurement Type | Meas Type |
| Mechanism of Action | Mechanism of Action |
| Model component | Model Comp |
| Morphologic abnormality | Morph Abnormality |
| MS-DRG | Diagnostic Category |
| Namespace concept | Namespace Concept |
| Note Type | Note Type |
| Observable entity | Observable Entity |
| Observation Period Type | Obs Period Type |
| Observation Type | Observation Type |
| OMOP DOI cohort | Drug Cohort |
| OMOP HOI cohort | Condition Cohort |
| OPCS-4 | Procedure |
| Organism | Organism |
| Patient Status | Patient Status |
| Pharmaceutical / biologic product | Pharma/Biol Product |
| Pharmaceutical Preparations | Pharma Preparation |
| Pharmacokinetics | PK |
| Pharmacologic Class | Pharmacologic Class |
| Physical force | Physical Force |
| Physical object | Physical Object |
| Physiologic Effect | Physiologic Effect |
| Place of Service | Place of Service |
| Preferred Term | PT |
| Procedure | Procedure |
| Procedure Occurrence Type | Procedure Occur Type |
| Qualifier value | Qualifier Value |
| Race | Race |
| Record artifact | Record Artifact |
| Revenue Code | Revenue Code |
| Sex | Gender |
| Social context | Social Context |
| Special concept | Special Concept |
| Specimen | Specimen |
| Staging and scales | Staging / Scales |
| Standardized MedDRA Query | SMQ |
| Substance | Substance |
| System Organ Class | SOC |
| Therapeutic Class | Therapeutic Class |
| UCUM | Unit |
| UCUM Canonical | Canonical Unit |
| UCUM Custom | Unit |
| UCUM Standard | Unit |
| Undefined | Undefined |
| UNKNOWN | Undefined |
| VA Class | Drug Class |
| VA Drug Interaction | Drug Interaction |
| VA Product | Drug Product |
| Visit | Visit |
| Visit Type | Visit Type |
CONCEPT_RELATIONSHIP table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The CONCEPT_RELATIONSHIP table contains records that define direct relationships between any two Concepts and the nature or type of the relationship. Each type of a relationship is defined in the RELATIONSHIP table.
| Field | Required | Type | Description |
|---|---|---|---|
| concept_id_1 | Yes | integer | A foreign key to a Concept in the CONCEPT table associated with the relationship. Relationships are directional, and this field represents the source concept designation. |
| concept_id_2 | Yes | integer | A foreign key to a Concept in the CONCEPT table associated with the relationship. Relationships are directional, and this field represents the destination concept designation. |
| relationship_id | Yes | varchar(20) | A unique identifier to the type or nature of the Relationship as defined in the RELATIONSHIP table. |
| valid_start_date | Yes | date | The date when the instance of the Concept Relationship is first recorded. |
| valid_end_date | Yes | date | The date when the Concept Relationship became invalid because it was deleted or superseded (updated) by a new relationship. Default value is 31-Dec-2099. |
| invalid_reason | No | varchar(1) | Reason the relationship was invalidated. Possible values are 'D' (deleted), 'U' (replaced with an update) or NULL when valid_end_date has the default value. |
Conventions
- Relationships can generally be classified as hierarchical (parent-child) or non-hierarchical (lateral).
- All Relationships are directional, and each Concept Relationship is represented twice symmetrically within the CONCEPT_RELATIONSHIP table. For example, the two SNOMED concepts of ‘Acute myocardial infarction of the anterior wall’ and ‘Acute myocardial infarction’ have two Concept Relationships: 1- ‘Acute myocardial infarction of the anterior wall’ ‘Is a’ ‘Acute myocardial infarction’, and 2- ‘Acute myocardial infarction’ ‘Subsumes’ ‘Acute myocardial infarction of the anterior wall’.
- There is one record for each Concept Relationship connecting the same Concepts with the same relationship_id.
- Since all Concept Relationships exist with their mirror image (concept_id_1 and concept_id_2 swapped, and the relationship_id replaced by the reverse_relationship_id from the RELATIONSHIP table), it is not necessary to query for the existence of a relationship both in the concept_id_1 and concept_id_2 fields.
- Concept Relationships define direct relationships between Concepts. Indirect relationships through 3rd Concepts are not captured in this table. However, the CONCEPT_ANCESTOR table does this for hierachical relationships over several “generations” of direct relationships.
- In previous versions of the CDM, the relationship_id used to be a numerical identifier. See the RELATIONSHIP table.
RELATIONSHIP table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The RELATIONSHIP table provides a reference list of all types of relationships that can be used to associate any two concepts in the CONCEPT_RELATIONSHP table.
| Field | Required | Type | Description |
|---|---|---|---|
| relationship_id | Yes | varchar(20) | The type of relationship captured by the relationship record. |
| relationship_name | Yes | varchar(255) | The text that describes the relationship type. |
| is_hierarchical | Yes | varchar(1) | Defines whether a relationship defines concepts into classes or hierarchies. Values are 1 for hierarchical relationship or 0 if not. |
| defines_ancestry | Yes | varchar(1) | Defines whether a hierarchical relationship contributes to the concept_ancestor table. These are subsets of the hierarchical relationships. Valid values are 1 or 0. |
| reverse_relationship_id | Yes | varchar(20) | The identifier for the relationship used to define the reverse relationship between two concepts. |
| relationship_concept_id | Yes | integer | A foreign key that refers to an identifier in the CONCEPT table for the unique relationship concept. |
Conventions
- There is one record for each Relationship.
- Relationships are classified as hierarchical (parent-child) or non-hierarchical (lateral)
- They are used to determine which concept relationship records should be included in the computation of the CONCEPT_ANCESTOR table.
- The relationship_id field contains an alphanumerical identifier, that can also be used as the abbreviation of the Relationship.
- The relationship_name field contains the unabbreviated names of the Relationship.
- Relationships all exist symmetrically, i.e. in both direction. The relationship_id of the opposite Relationship is provided in the reverse_relationship_id field.
- Each Relationship also has an equivalent entry in the Concept table, which is recorded in the relationship_concept_id field. This is for purposes of creating a closed Information Model, where all entities in the OMOP CDM are covered by unique Concepts.
- Hierarchical Relationships are used to build a hierarchical tree out of the Concepts, which is recorded in the CONCEPT_ANCESTOR table. For example, “has_ingredient” is a Relationship between Concepst of the Concept Class 'Clinical Drug' and those of 'Ingredient', and all Ingredients can be classified as the “parental” hierarchical Concepts for the drug products they are part of. All 'Is a' Relationships are hierarchical.
- Relationships, also hierarchical, can be between Concepts within the same Vocabulary or those adopted from different Vocabulary sources.
- In past versions of the RELATIONSHIP table, the relationship_id used to be a numerical value. A conversion table between these old and new IDs is given below:
| relationship_id previously | relationship_id Version 5 |
|---|---|
| 1 | LOINC replaced by |
| 2 | Has precise ing |
| 3 | Has tradename |
| 4 | RxNorm has dose form |
| 5 | Has form |
| 6 | RxNorm has ing |
| 7 | Constitutes |
| 8 | Contains |
| 9 | Reformulation of |
| 10 | Subsumes |
| 11 | NDFRT has dose form |
| 12 | Induces |
| 13 | May diagnose |
| 14 | Has physio effect |
| 15 | Has CI physio effect |
| 16 | NDFRT has ing |
| 17 | Has CI chem class |
| 18 | Has MoA |
| 19 | Has CI MoA |
| 20 | Has PK |
| 21 | May treat |
| 22 | CI to |
| 23 | May prevent |
| 24 | Has metabolites |
| 25 | Has metabolism |
| 26 | May be inhibited by |
| 27 | Has chem structure |
| 28 | NDFRT - RxNorm eq |
| 29 | Has recipient cat |
| 30 | Has proc site |
| 31 | Has priority |
| 32 | Has pathology |
| 33 | Has part of |
| 34 | Has severity |
| 35 | Has revision status |
| 36 | Has access |
| 37 | Has occurrence |
| 38 | Has method |
| 39 | Has laterality |
| 40 | Has interprets |
| 41 | Has indir morph |
| 42 | Has indir device |
| 43 | Has specimen |
| 44 | Has interpretation |
| 45 | Has intent |
| 46 | Has focus |
| 47 | Has manifestation |
| 48 | Has active ing |
| 49 | Has finding site |
| 50 | Has episodicity |
| 51 | Has dir subst |
| 52 | Has dir morph |
| 53 | Has dir device |
| 54 | Has component |
| 55 | Has causative agent |
| 56 | Has asso morph |
| 57 | Has asso finding |
| 58 | Has measurement |
| 59 | Has property |
| 60 | Has scale type |
| 61 | Has time aspect |
| 62 | Has specimen proc |
| 63 | Has specimen source |
| 64 | Has specimen morph |
| 65 | Has specimen topo |
| 66 | Has specimen subst |
| 67 | Has due to |
| 68 | Has relat context |
| 69 | Has dose form |
| 70 | Occurs after |
| 71 | Has asso proc |
| 72 | Has dir proc site |
| 73 | Has indir proc site |
| 74 | Has proc device |
| 75 | Has proc morph |
| 76 | Has finding context |
| 77 | Has proc context |
| 78 | Has temporal context |
| 79 | Findinga sso with |
| 80 | Has surgical appr |
| 81 | Using device |
| 82 | Using energy |
| 83 | Using subst |
| 84 | Using acc device |
| 85 | Has clinical course |
| 86 | Has route of admin |
| 87 | Using finding method |
| 88 | Using finding inform |
| 92 | ICD9P - SNOMED eq |
| 93 | CPT4 - SNOMED cat |
| 94 | CPT4 - SNOMED eq |
| 125 | MedDRA - SNOMED eq |
| 126 | Has FDA-appr ind |
| 127 | Has off-label ind |
| 129 | Has CI |
| 130 | ETC - RxNorm |
| 131 | ATC - RxNorm |
| 132 | SMQ - MedDRA |
| 135 | LOINC replaces |
| 136 | Precise ing of |
| 137 | Tradename of |
| 138 | RxNorm dose form of |
| 139 | Form of |
| 140 | RxNorm ing of |
| 141 | Consists of |
| 142 | Contained in |
| 143 | Reformulated in |
| 144 | Is a |
| 145 | NDFRT dose form of |
| 146 | Induced by |
| 147 | Diagnosed through |
| 148 | Physiol effect by |
| 149 | CI physiol effect by |
| 150 | NDFRT ing of |
| 151 | CI chem class of |
| 152 | MoA of |
| 153 | CI MoA of |
| 154 | PK of |
| 155 | May be treated by |
| 156 | CI by |
| 157 | May be prevented by |
| 158 | Metabolite of |
| 159 | Metabolism of |
| 160 | Inhibits effect |
| 161 | Chem structure of |
| 162 | RxNorm - NDFRT eq |
| 163 | Recipient cat of |
| 164 | Proc site of |
| 165 | Priority of |
| 166 | Pathology of |
| 167 | Part of |
| 168 | Severity of |
| 169 | Revision status of |
| 170 | Access of |
| 171 | Occurrence of |
| 172 | Method of |
| 173 | Laterality of |
| 174 | Interprets of |
| 175 | Indir morph of |
| 176 | Indir device of |
| 177 | Specimen of |
| 178 | Interpretation of |
| 179 | Intent of |
| 180 | Focus of |
| 181 | Manifestation of |
| 182 | Active ing of |
| 183 | Finding site of |
| 184 | Episodicity of |
| 185 | Dir subst of |
| 186 | Dir morph of |
| 187 | Dir device of |
| 188 | Component of |
| 189 | Causative agent of |
| 190 | Asso morph of |
| 191 | Asso finding of |
| 192 | Measurement of |
| 193 | Property of |
| 194 | Scale type of |
| 195 | Time aspect of |
| 196 | Specimen proc of |
| 197 | Specimen identity of |
| 198 | Specimen morph of |
| 199 | Specimen topo of |
| 200 | Specimen subst of |
| 201 | Due to of |
| 202 | Relat context of |
| 203 | Dose form of |
| 204 | Occurs before |
| 205 | Asso proc of |
| 206 | Dir proc site of |
| 207 | Indir proc site of |
| 208 | Proc device of |
| 209 | Proc morph of |
| 210 | Finding context of |
| 211 | Proc context of |
| 212 | Temporal context of |
| 213 | Asso with finding |
| 214 | Surgical appr of |
| 215 | Device used by |
| 216 | Energy used by |
| 217 | subst used by |
| 218 | Acc device used by |
| 219 | Clinical course of |
| 220 | Route of admin of |
| 221 | Finding method of |
| 222 | Finding inform of |
| 226 | SNOMED - ICD9P eq |
| 227 | SNOMED cat - CPT4 |
| 228 | SNOMED - CPT4 eq |
| 239 | SNOMED - MedDRA eq |
| 240 | Is FDA-appr ind of |
| 241 | Is off-label ind of |
| 243 | Is CI of |
| 244 | RxNorm - ETC |
| 245 | RxNorm - ATC |
| 246 | MedDRA - SMQ |
| 247 | Ind/CI - SNOMED |
| 248 | SNOMED - ind/CI |
| 275 | Has therap class |
| 276 | Therap class of |
| 277 | Drug-drug inter for |
| 278 | Has drug-drug inter |
| 279 | Has pharma prep |
| 280 | Pharma prep in |
| 281 | Inferred class of |
| 282 | Has inferred class |
| 283 | SNOMED proc - HCPCS |
| 284 | HCPCS - SNOMED proc |
| 285 | RxNorm - NDFRT name |
| 286 | NDFRT - RxNorm name |
| 287 | ETC - RxNorm name |
| 288 | RxNorm - ETC name |
| 289 | ATC - RxNorm name |
| 290 | RxNorm - ATC name |
| 291 | HOI - SNOMED |
| 292 | SNOMED - HOI |
| 293 | DOI - RxNorm |
| 294 | RxNorm - DOI |
| 295 | HOI - MedDRA |
| 296 | MedDRA - HOI |
| 297 | NUCC - CMS Specialty |
| 298 | CMS Specialty - NUCC |
| 299 | DRG - MS-DRG eq |
| 300 | MS-DRG - DRG eq |
| 301 | DRG - MDC cat |
| 302 | MDC cat - DRG |
| 303 | Visit cat - PoS |
| 304 | PoS - Visit cat |
| 305 | VAProd - NDFRT |
| 306 | NDFRT - VAProd |
| 307 | VAProd - RxNorm eq |
| 308 | RxNorm - VAProd eq |
| 309 | RxNorm replaced by |
| 310 | RxNorm replaces |
| 311 | SNOMED replaced by |
| 312 | SNOMED replaces |
| 313 | ICD9P replaced by |
| 314 | ICD9P replaces |
| 315 | Multilex has ing |
| 316 | Multilex ing of |
| 317 | RxNorm - Multilex eq |
| 318 | Multilex - RxNorm eq |
| 319 | Multilex ing - class |
| 320 | Class - Multilex ing |
| 321 | Maps to |
| 322 | Mapped from |
| 325 | Map includes child |
| 326 | Included in map from |
| 327 | Map excludes child |
| 328 | Excluded in map from |
| 345 | UCUM replaced by |
| 346 | UCUM replaces |
| 347 | Concept replaced by |
| 348 | Concept replaces |
| 349 | Concept same_as to |
| 350 | Concept same_as from |
| 351 | Concept alt_to to |
| 352 | Concept alt_to from |
| 353 | Concept poss_eq to |
| 354 | Concept poss_eq from |
| 355 | Concept was_a to |
| 356 | Concept was_a from |
| 357 | SNOMED meas - HCPCS |
| 358 | HCPCS - SNOMED meas |
| 359 | Domain subsumes |
| 360 | Is domain |
CONCEPT_SYNONYM table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The CONCEPT_SYNONYM table is used to store alternate names and descriptions for Concepts.
| Field | Required | Type | Description |
|---|---|---|---|
| concept_id | Yes | Integer | A foreign key to the Concept in the CONCEPT table. |
| concept_synonym_name | Yes | varchar(1000) | The alternative name for the Concept. |
| language_concept_id | Yes | integer | A foreign key to a Concept representing the language. |
Conventions
- The concept_name field contains a valid Synonym of a concept, including the description in the concept_name itself. I.e. each Concept has at least one Synonym in the CONCEPT_SYNONYM table. As an example, for a SNOMED-CT Concept, if the fully specified name is stored as the concept_name of the CONCEPT table, then the Preferred Term and Synonyms associated with the Concept are stored in the CONCEPT_SYNONYM table.
- Only Synonyms that are active and current are stored in the CONCEPT_SYNONYM table. Tracking synonym/description history and mapping of obsolete synonyms to current Concepts/Synonyms is out of scope for the Standard Vocabularies.
- Currently, only English Synonyms are included.
CONCEPT_ANCESTOR table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
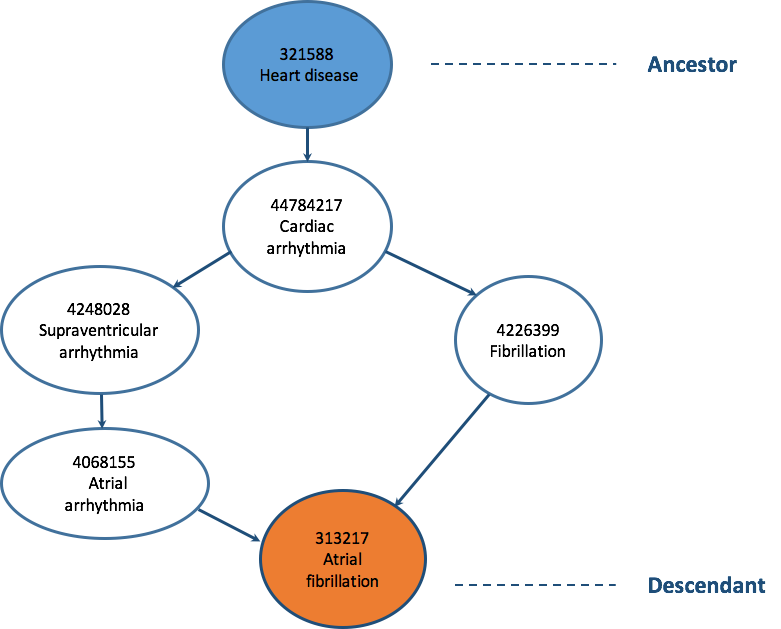
The CONCEPT_ANCESTOR table is designed to simplify observational analysis by providing the complete hierarchical relationships between Concepts. Only direct parent-child relationships between Concepts are stored in the CONCEPT_RELATIONSHIP table. To determine higher level ancestry connections, all individual direct relationships would have to be navigated at analysis time. The CONCEPT_ANCESTOR table includes records for all parent-child relationships, as well as grandparent-grandchild relationships and those of any other level of lineage. Using the CONCEPT_ANCESTOR table allows for querying for all descendants of a hierarchical concept. For example, drug ingredients and drug products are all descendants of a drug class ancestor.
This table is entirely derived from the CONCEPT, CONCEPT_RELATIONSHIP and RELATIONSHIP tables.
| Field | Required | Type | Description |
|---|---|---|---|
| ancestor_concept_id | Yes | integer | A foreign key to the concept in the concept table for the higher-level concept that forms the ancestor in the relationship. |
| descendant_concept_id | Yes | integer | A foreign key to the concept in the concept table for the lower-level concept that forms the descendant in the relationship. |
| min_levels_of_separation | Yes | integer | The minimum separation in number of levels of hierarchy between ancestor and descendant concepts. This is an attribute that is used to simplify hierarchic analysis. |
| max_levels_of_separation | Yes | integer | The maximum separation in number of levels of hierarchy between ancestor and descendant concepts. This is an attribute that is used to simplify hierarchic analysis. |
A path between two concepts can be characterized by the sequence of relationships that need to be traversed in order to reach a descendant concept from an ancestor concept.
For example, for concepts
| descendant_concept_id | descendant_concept_name | ancestor_concept_id | ancestor_concept_name | min_levels_of_separation | max_levels_of_separation |
|---|---|---|---|---|---|
| 313217 | Atrial fibrillation | 321588 | Heart disease | 3 | 4 |
the shortest path in concept_relationship will be :
313217 Atrial fibrillation Is a 4226399 Fibrillation Is a 44784217 Cardiac arrhythmia Is a 321588 Heart disease
the longest:
313217 Atrial fibrillation Is a 4068155 Atrial arrhythmia Is a 4248028 Supraventricular arrhythmia Is a 44784217 Cardiac arrhythmia Is a 321588 Heart disease
Conventions
- Each concept is also recorded as an ancestor of itself.
- Only valid and Standard Concepts participate in the CONCEPT_ANCESTOR table. It is not possible to find ancestors or descendants of deprecated or Source Concepts.
- Usually, only Concepts of the same Domain are connected through records of the CONCEPT_ANCESTOR table, but there might be exceptions.
SOURCE_TO_CONCEPT_MAP table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The source to concept map table is a legacy data structure within the OMOP Common Data Model, recommended for use in ETL processes to maintain local source codes which are not available as Concepts in the Standardized Vocabularies, and to establish mappings for each source code into a Standard Concept as target_concept_ids that can be used to populate the Common Data Model tables. The SOURCE_TO_CONCEPT_MAP table is no longer populated with content within the Standardized Vocabularies published to the OMOP community.
| Field | Required | Type | Description |
|---|---|---|---|
| source_code | Yes | varchar(50) | The source code being translated into a Standard Concept. |
| source_concept_id | Yes | integer | A foreign key to the Source Concept that is being translated into a Standard Concept. |
| source_vocabulary_id | No | varchar(20) | A foreign key to the VOCABULARY table defining the vocabulary of the source code that is being translated to a Standard Concept. |
| source_code_description | Yes | varchar(255) | An optional description for the source code. This is included as a convenience to compare the description of the source code to the name of the concept. |
| target_concept_id | Yes | integer | A foreign key to the target Concept to which the source code is being mapped. |
| target_vocabulary_id | Yes | varchar(20) | A foreign key to the VOCABULARY table defining the vocabulary of the target Concept. |
| valid_start_date | Yes | date | The date when the mapping instance was first recorded. |
| valid_end_date | Yes | date | The date when the mapping instance became invalid because it was deleted or superseded (updated) by a new relationship. Default value is 31-Dec-2099. |
| invalid_reason | No | varchar(1) | Reason the mapping instance was invalidated. Possible values are D (deleted), U (replaced with an update) or NULL when valid_end_date has the default value. |
Conventions
- This table is no longer used to distribute mapping information between source codes and Standard Concepts for the Standard Vocabularies. Instead, the CONCEPT_RELATIONSHIP table is used for this purpose, using the relationship_id='Maps to'.
- However, this table can still be used for the translation of local source codes into Standard Concepts.
- Note: This table should not be used to translate source codes to Source Concepts. The source code of a Source Concept is captured in its concept_code field. If the source codes used in a given database do not follow correct formatting the ETL will have to perform this translation. For example, if ICD-9-CM codes are recorded without a dot the ETL will have to perform a lookup function that allows identifying the correct ICD-9-CM Source Concept (with the dot in the concept_code field).
- The source_concept_id, or the combination of the fields source_code and the source_vocabulary_id uniquely identifies the source information. It is the equivalent to the concept_id_1 field in the CONCEPT_RELATIONSHIP table.
- If there is no source_concept_id available because the source codes are local and not supported by the Standard Vocabulary, the content of the field is 0 (zero, not null) encoding an undefined concept. However, local Source Concepts are established (concept_id values above 2,000,000,000).
- The source_code_description contains an optional description of the source code.
- The target_concept_id contains the Concept the source code is mapped to. It is equivalent to the concept_id_2 in the CONCEPT_RELATIONSHIP table
- The target_vocabulary_id field contains the vocabulary_id of the target concept. It is a duplication of the same information in the CONCEPT record of the Target Concept.
- The fields valid_start_date, valid_end_date and invalid_reason are used to define the life cycle of the mapping information. Invalid mapping records should not be used for mapping information.
DRUG_STRENGTH table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table had the field denominator_value added with version 5.0.1 (5-Apr-2016) of the OMOP CDM.
The DRUG_STRENGTH table contains structured content about the amount or concentration and associated units of a specific ingredient contained within a particular drug product. This table is supplemental information to support standardized analysis of drug utilization.
| Field | Required | Type | Description |
|---|---|---|---|
| drug_concept_id | Yes | integer | A foreign key to the Concept in the CONCEPT table representing the identifier for Branded Drug or Clinical Drug Concept. |
| ingredient_concept_id | Yes | integer | A foreign key to the Concept in the CONCEPT table, representing the identifier for drug Ingredient Concept contained within the drug product. |
| amount_value | No | float | The numeric value associated with the amount of active ingredient contained within the product. |
| amount_unit_concept_id | No | integer | A foreign key to the Concept in the CONCEPT table representing the identifier for the Unit for the absolute amount of active ingredient. |
| numerator_value | No | float | The numeric value associated with the concentration of the active ingredient contained in the product |
| numerator_unit_concept_id | No | integer | A foreign key to the Concept in the CONCEPT table representing the identifier for the numerator Unit for the concentration of active ingredient. |
| denominator_value (V5.0.1) | No | float | The amount of total liquid (or other divisible product, such as ointment, gel, spray, etc.). |
| denominator_unit_concept_id | No | integer | A foreign key to the Concept in the CONCEPT table representing the identifier for the denominator Unit for the concentration of active ingredient. |
| valid_start_date | Yes | date | The date when the Concept was first recorded. The default value is 1-Jan-1970. |
| valid_end_date | Yes | date | The date when the concept became invalid because it was deleted or superseded (updated) by a new Concept. The default value is 31-Dec-2099. |
| invalid_reason | No | varchar(1) | Reason the concept was invalidated. Possible values are 'D' (deleted), 'U' (replaced with an update) or NULL when valid_end_date has the default value. |
Conventions
- The DRUG_STRENGTH table contains information for each active (non-deprecated) standard drug concept.
- A drug which contains multiple active Ingredients will result in multiple DRUG_STRENGTH records, one for each active ingredient.
- Ingredient strength information is provided either as absolute amount (usually for solid formulations) or as concentration (usually for liquid formulations).
- If the absolute amount is provided (for example, 'Acetaminophen 5 MG Tablet') the amount_value and amount_unit_concept_id are used to define this content (in this case 5 and 'MG').
- If the concentration is provided (for example 'Acetaminophen 48 MG/ML Oral Solution') the numerator_value in combination with the numerator_unit_concept_id and denominator_unit_concept_id are used to define this content (in this case 48, 'MG' and 'ML').
- In case of Quantified Clinical or Branded Drugs the denominator_value contains the total amount of the solution (not the amount of the ingredient). In all other drug concept classes the denominator amount is NULL because the concentration is always normalized to the unit of the denominator. So, a product containing 960 mg in 20 mL is provided as 48 mg/mL in the Clinical Drug and Clinical Drug Component, while as a Quantified Clinical Drug it is written as 960 mg/20 mL.
- If the strength is provided in % (volume or mass-percent are not distinguished) it is stored in the numerator_value/numerator_unit_concept_id field combination, with both the denominator_value and denominator_unit_concept_id set to NULL. If it is a Quantified Drug the total amount of drug is provided in the denominator_value/denominator_unit_concept_id pair. E.g., the 30 G Isoconazole 2% Topical Cream is provided as 2% / in Clinical Drug and Clinical Drug Component, and as 2% /30 G.
- Sometimes, one Ingredient is listed with different units within the same drug. This is very rare, and usually this happens if there are more than one Precise Ingredient. For example, 'Penicillin G, Benzathine 150000 UNT/ML / Penicillin G, Procaine 150000 MEQ/ML Injectable Suspension' contains Penicillin G in two different forms.
- Sometimes, different ingredients in liquid drugs are listed with different units in the denominator_unit_concept_id. This is usually the case if the ingredients are liquids themselves (concentration provided as mL/mL) or solid substances (mg/mg). In these cases, the general assumptions is made that the density of the drug is that of water, and one can assume 1 g = 1 mL.
- All Drug vocabularies containing Standard Concepts have entries in the DRUG_STRENGTH table.
COHORT_DEFINITION table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The COHORT_DEFINITION table contains records defining a Cohort derived from the data through the associated description and syntax and upon instantiation (execution of the algorithm) placed into the COHORT table. Cohorts are a set of subjects that satisfy a given combination of inclusion criteria for a duration of time. The COHORT_DEFINITION table provides a standardized structure for maintaining the rules governing the inclusion of a subject into a cohort, and can store operational programming code to instantiate the cohort within the OMOP Common Data Model.
| Field | Required | Type | Description |
|---|---|---|---|
| cohort_definition_id | Yes | integer | A unique identifier for each Cohort. |
| cohort_definition_name | Yes | varchar(255) | A short description of the Cohort. |
| cohort_definition_description | No | CLOB | A complete description of the Cohort definition |
| definition_type_concept_id | Yes | integer | Type defining what kind of Cohort Definition the record represents and how the syntax may be executed |
| cohort_definition_syntax | No | CLOB | Syntax or code to operationalize the Cohort definition |
| subject_concept_id | Yes | integer | A foreign key to the Concept to which defines the domain of subjects that are members of the cohort (e.g., Person, Provider, Visit). |
| cohort_instantiation_date | No | Date | A date to indicate when the Cohort was instantiated in the COHORT table |
Conventions
- The cohort_definition_syntax does not prescribe any specific syntax or programming language. Typically, it would be any flavor SQL, a cohort definition language, or a free-text description of the algorithm.
- The subject_concept_id determines what the individual subjects or entities of the Cohort consists of. In most cases, that would be a Person (patient). But cohorts could also be constructed for Providers, Visits or any other Domain. Note that the Domain is not codified using the alphanumerical domain_id like in the CONCEPT table. Instead, the corresponding Concept is used. The Concepts for each domain can be obtained from the DOMAIN table in the domain_concept_id.
ATTRIBUTE_DEFINITION table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The ATTRIBUTE_DEFINITION table contains records defining Attributes, or covariates, to members of a Cohort through an associated description and syntax and upon instantiation (execution of the algorithm) placed into the COHORT_ATTRIBUTE table. Attributes are derived elements that can be selected or calculated for a subject in a Cohort. The ATTRIBUTE_DEFINITION table provides a standardized structure for maintaining the rules governing the calculation of covariates for a subject in a Cohort, and can store operational programming code to instantiate the Attributes for a given Cohort within the OMOP Common Data Model.
| Field | Required | Type | Description |
|---|---|---|---|
| attribute_definition_id | Yes | integer | A unique identifier for each Attribute. |
| attribute_name | Yes | varchar(255) | A short description of the Attribute. |
| attribute_description | No | CLOB | A complete description of the Attribute definition |
| attribute_type_concept_id | Yes | integer | Type defining what kind of Attribute Definition the record represents and how the syntax may be executed |
| attribute_syntax | No | CLOB | Syntax or code to operationalize the Attribute definition |
Conventions
- Like the definition syntax field for the COHORT_DEFINITION table, the attribute_definition_syntax does not prescribe any specific syntax or programming language. Typically, it would be any flavor SQL, or a cohort definition language, or a free-text description of the algorithm.
- The Attribute Definition is generic and not necessarily related to a specific Cohort Definition, however the instantiated Attribute is linked to the Cohort records (see below the COHORT table. For example, the Attribute “Age” can be defined as the amount of time between the cohort_start_date of the COHORT table and the year_of_birth, month_of_birth and day_of_birth of the PERSON table. Thus, such a Attribute Definition can be applied and instantiated with any Cohort, as long as it is applied to a Cohort of the same Domain (Person in this case), as it is defined in the subject_concept_id in the COHORT_DEFINITION table.
CDM_SOURCE table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The CDM_SOURCE table contains detail about the source database and the process used to transform the data into the OMOP Common Data Model.
| Field | Required | Type | Description |
|---|---|---|---|
| cdm_source_name | Yes | varchar(255) | The full name of the source |
| cdm_source_abbreviation | No | varchar(25) | An abbreviation of the name |
| cdm_holder | No | varchar(255) | The name of the organization responsible for the development of the CDM instance |
| source_description | No | CLOB | A description of the source data origin and purpose for collection. The description may contain a summary of the period of time that is expected to be covered by this dataset. |
| source_documentation_reference | No | varchar(255) | URL or other external reference to location of source documentation |
| cdm_etl _reference | No | varchar(255) | URL or other external reference to location of ETL specification documentation and ETL source code |
| source_release_date | No | date | The date for which the source data are most current, such as the last day of data capture |
| cdm_release_date | No | date | The date when the CDM was instantiated |
| cdm_version | No | varchar(10) | The version of CDM used |
| vocabulary_version | No | varchar(20) | The version of the vocabulary used |
Conventions
- If a source database is derived from multiple data feeds, the integration of those disparate sources is expected to be documented in the ETL specifications. The source information on each of the databases can be represented as separate records in the CDM_SOURCE table.
- Currently, there is no mechanism to link individual records in the CDM tables to their source record in the CDM_SOURCE table.
- The version of the vocabulary can be obtained from the vocabulary_name field in the VOCABULARY table for the record where vocabulary_id='None'.
Standardized Clinical Data Tables
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
These tables contain the core information about the clinical events that occurred longitudinally during valid Observation Periods for each Person, as well as demographic information for the Person. Below provides an entity-relationship diagram highlighting the tables within the Standardized Clinical Data portion of the OMOP Common Data Model:

PERSON table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table changed in version 5.1 of the OMOP CDM. The name of the field time_of_birth was changed to birth_datetime.
The Person Domain contains records that uniquely identify each patient in the source data who is time at-risk to have clinical observations recorded within the source systems.
| Field | Required | Type | Description |
|---|---|---|---|
| person_id | Yes | integer | A unique identifier for each person. |
| gender_concept_id | Yes | integer | A foreign key that refers to an identifier in the CONCEPT table for the unique gender of the person. |
| year_of_birth | Yes | integer | The year of birth of the person. For data sources with date of birth, the year is extracted. For data sources where the year of birth is not available, the approximate year of birth is derived based on any age group categorization available. |
| month_of_birth | No | integer | The month of birth of the person. For data sources that provide the precise date of birth, the month is extracted and stored in this field. |
| day_of_birth | No | integer | The day of the month of birth of the person. For data sources that provide the precise date of birth, the day is extracted and stored in this field. |
| birth_datetime | No | datetime | The date and time of birth of the person. |
| race_concept_id | Yes | integer | A foreign key that refers to an identifier in the CONCEPT table for the unique race of the person. |
| ethnicity_concept_id | Yes | integer | A foreign key that refers to the standard concept identifier in the Standardized Vocabularies for the ethnicity of the person. |
| location_id | No | integer | A foreign key to the place of residency for the person in the location table, where the detailed address information is stored. |
| provider_id | No | integer | A foreign key to the primary care provider the person is seeing in the provider table. |
| care_site_id | No | integer | A foreign key to the site of primary care in the care_site table, where the details of the care site are stored. |
| person_source_value | No | varchar(50) | An (encrypted) key derived from the person identifier in the source data. This is necessary when a use case requires a link back to the person data at the source dataset. |
| gender_source_value | No | varchar(50) | The source code for the gender of the person as it appears in the source data. The person’s gender is mapped to a standard gender concept in the Standardized Vocabularies; the original value is stored here for reference. |
| gender_source_concept_id | No | Integer | A foreign key to the gender concept that refers to the code used in the source. |
| race_source_value | No | varchar(50) | The source code for the race of the person as it appears in the source data. The person race is mapped to a standard race concept in the Standardized Vocabularies and the original value is stored here for reference. |
| race_source_concept_id | No | Integer | A foreign key to the race concept that refers to the code used in the source. |
| ethnicity_source_value | No | varchar(50) | The source code for the ethnicity of the person as it appears in the source data. The person ethnicity is mapped to a standard ethnicity concept in the Standardized Vocabularies and the original code is, stored here for reference. |
| ethnicity_source_concept_id | No | Integer | A foreign key to the ethnicity concept that refers to the code used in the source. |
Conventions
- All tables representing patient-related Domains have a foreign-key reference to the person_id field in the PERSON table.
- Each person record has associated demographic attributes which are assumed to be constant for the patient throughout the course of their periods of observation. For example, the location or gender is expected to have a unique value per person, even though in life these data may change over time.
- Valid Gender, Race and Ethnicity Concepts each belong to their own Domain.
- Ethnicity in the OMOP CDM follows the OMB Standards for Data on Race and Ethnicity: Only distinctions between Hispanics and Non-Hispanics are made.
- Additional information is stored through references to other tables, such as the home address (location_id) or the primary care provider.
- The Provider refers to the primary care provider (General Practitioner).
- The Care Site refers to where the Provider typically provides the primary care.
OBSERVATION_PERIOD table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The OBSERVATION_PERIOD table contains records which uniquely define the spans of time for which a Person is at-risk to have clinical events recorded within the source systems, even if no events in fact are recorded (healthy patient with no healthcare interactions).
| Field | Required | Type | Description |
|---|---|---|---|
| observation_period_id | Yes | integer | A unique identifier for each observation period. |
| person_id | Yes | integer | A foreign key identifier to the person for whom the observation period is defined. The demographic details of that person are stored in the person table. |
| observation_period_start_date | Yes | date | The start date of the observation period for which data are available from the data source. |
| observation_period_end_date | Yes | date | The end date of the observation period for which data are available from the data source. |
| period_type_concept_id | Yes | Integer | A foreign key identifier to the predefined concept in the Standardized Vocabularies reflecting the source of the observation period information |
Conventions
- One Person may have one or more disjoint observation periods, during which times analyses may assume that clinical events would be captured if observed, and outside of which no clinical events may be recorded.
- Each Person can have more than one valid OBSERVATION_PERIOD record, but no two observation periods can overlap in time for a given person.
- As a general assumption, during an Observation Period any clinical event that happens to the patient is expected to be recorded. Conversely, the absence of data indicates that no clinical events occurred to the patient.
- No clinical data are valid outside an active Observation Period. Clinical data that refer to a time outside (diagnoses of previous conditions such as “Old MI” or medical history) of an active Observation Period are recorded as Observations. The date of the Observation is the first day of the first Observation Period of a patient.
- For claims data, observation periods are inferred from the enrollment periods to a health benefit plan.
- For EHR data, the observation period cannot be determined explicitly, because patients usually do not announce their departure from a certain healthcare provider. The ETL will have to apply some heuristic to make a reasonable guess on what the observation_period should be. Refer to the ETL documentation for details.
SPECIMEN
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table changed in version 5.1 of the OMOP CDM. The field specimen_datetime was added.
The specimen domain contains the records identifying biological samples from a person.
| Field | Required | Type | Description |
|---|---|---|---|
| specimen_id | Yes | integer | A unique identifier for each specimen. |
| person_id | Yes | integer | A foreign key identifier to the Person for whom the Specimen is recorded. |
| specimen_concept_id | Yes | integer | A foreign key referring to a Standard Concept identifier in the Standardized Vocabularies for the Specimen. |
| specimen_type_concept_id | Yes | integer | A foreign key referring to the Concept identifier in the Standardized Vocabularies reflecting the system of record from which the Specimen was represented in the source data. |
| specimen_date | Yes | date | The date the specimen was obtained from the Person. |
| specimen_datetime | No | datetime | The date and time on the date when the Specimen was obtained from the person. |
| quantity | No | float | The amount of specimen collection from the person during the sampling procedure. |
| unit_concept_id | No | integer | A foreign key to a Standard Concept identifier for the Unit associated with the numeric quantity of the Specimen collection. |
| anatomic_site_concept_id | No | integer | A foreign key to a Standard Concept identifier for the anatomic location of specimen collection. |
| disease_status_concept_id | No | integer | A foreign key to a Standard Concept identifier for the Disease Status of specimen collection. |
| specimen_source_id | No | varchar(50) | The Specimen identifier as it appears in the source data. |
| specimen_source_value | No | varchar(50) | The Specimen value as it appears in the source data. This value is mapped to a Standard Concept in the Standardized Vocabularies and the original code is, stored here for reference. |
| unit_source_value | No | varchar(50) | The information about the Unit as detailed in the source. |
| anatomic_site_source_value | No | varchar(50) | The information about the anatomic site as detailed in the source. |
| disease_status_source_value | No | varchar(50) | The information about the disease status as detailed in the source. |
Conventions
- Anatomic site is coded at the most specific level of granularity possible, such that higher level classifications can be derived using the Standardized Vocabularies.
DEATH table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table changed in version 5.1 of the OMOP CDM. The death_datetime was added.
The death domain contains the clinical event for how and when a Person dies. A person can have up to one record if the source system contains evidence about the Death, such as:
- Condition Code in the Header or Detail information of claims
- Status of enrollment into a health plan
- Explicit record in EHR data
| Field | Required | Type | Description |
|---|---|---|---|
| person_id | Yes | integer | A foreign key identifier to the deceased person. The demographic details of that person are stored in the person table. |
| death_date | Yes | date | The date the person was deceased. If the precise date including day or month is not known or not allowed, December is used as the default month, and the last day of the month the default day. |
| death_datetime | No | datetime | The date and time the person was deceased. If the precise date including day or month is not known or not allowed, December is used as the default month, and the last day of the month the default day. |
| death_type_concept_id | Yes | integer | A foreign key referring to the predefined concept identifier in the Standardized Vocabularies reflecting how the death was represented in the source data. |
| cause_concept_id | No | integer | A foreign key referring to a standard concept identifier in the Standardized Vocabularies for conditions. |
| cause_source_value | No | varchar(50) | The source code for the cause of death as it appears in the source data. This code is mapped to a standard concept in the Standardized Vocabularies and the original code is, stored here for reference. |
| cause_source_concept_id | No | integer | A foreign key to the concept that refers to the code used in the source. Note, this variable name is abbreviated to ensure it will be allowable across database platforms. |
Conventions
- Living patients should not contain any information in the DEATH table.
- Each Person may have more than one record of death in the source data. It is the task of the ETL to pick the most plausible or most accurate records to be aggregated and stored as a single record in the DEATH table.
- If the Death Date cannot be precisely determined from the data, the best approximation should be used.
- Valid Concepts for the cause_concept_id have domain_id='Condition'.
VISIT_OCCURRENCE table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table changed version 5.1 of the OMOP CDM. The fields visit_start_datetime and visit_end_datetime were added. For 5.0.1, the fields admitting_source_concept_id, discharge_to_concept_id, admitting_source_value and discharge_to_source_value were added.
The VISIT_OCCURRENCE table contains the spans of time a Person continuously receives medical services from one or more providers at a Care Site in a given setting within the health care system. Visits are classified into 4 settings: outpatient care, inpatient confinement, emergency room, and long-term care. Persons may transition between these settings over the course of an episode of care (for example, treatment of a disease onset).
| Field | Required | Type | Description |
|---|---|---|---|
| visit_occurrence_id | Yes | integer | A unique identifier for each Person's visit or encounter at a healthcare provider. |
| person_id | Yes | integer | A foreign key identifier to the Person for whom the visit is recorded. The demographic details of that Person are stored in the PERSON table. |
| visit_concept_id | Yes | integer | A foreign key that refers to a visit Concept identifier in the Standardized Vocabularies. |
| visit_start_date | Yes | date | The start date of the visit. |
| visit_start_datetime | No | datetime | The date and time of the visit started. |
| visit_end_date | Yes | date | The end date of the visit. If this is a one-day visit the end date should match the start date. |
| visit_end_datetime | No | datetime | The date and time of the visit end. |
| visit_type_concept_id | Yes | Integer | A foreign key to the predefined Concept identifier in the Standardized Vocabularies reflecting the type of source data from which the visit record is derived. |
| provider_id | No | integer | A foreign key to the provider in the provider table who was associated with the visit. |
| care_site_id | No | integer | A foreign key to the care site in the care site table that was visited. |
| admitting_source_concept_id | No | integer | A foreign key to the predefined concept in the Place of Service Vocabulary reflecting the admitting source for a visit. |
| discharge_to_concept_id | No | integer | A foreign key to the predefined concept in the Place of Service Vocabulary reflecting the discharge disposition (destination) for a visit. |
| preceding_visit_occurrence_id | No | integer | A foreign key to the VISIT_OCCURRENCE table record of the visit immediately preceding this visit. |
| visit_source_value | No | string(50) | The source code for the visit as it appears in the source data. |
| visit_source_concept_id | No | Integer | A foreign key to a Concept that refers to the code used in the source. |
| admitting_source_value | No | string(50) | The source code for the admitting source as it appears in the source data. |
| discharge_to_source_value | No | string(50) | The source code for the discharge disposition as it appears in the source data. |
Conventions
- A Visit Occurrence is recorded for each visit to a healthcare facility.
- Valid Visit Concepts belong to the “Visit” domain.
- Standard Visit Concepts are defined as Inpatient Visit, Outpatient Visit, Emergency Room Visit, Long Term Care Visit and combined ER and Inpatient Visit. The latter is necessary because it is close to impossible to separate the two in many EHR system, treating them interchangeably. To annotate this correctly, the visit concept “Emergency Room and Inpatient Visit” (concept_id=262) should be used.
- Handling of death: In case when patient died during admission (Visit_Occurrence. discharge_disposition_concept_id = 4216643 ‘Patient died’), a record in the Death table should be created with death_type_concept_id = 44818516 (“EHR discharge status “Expired”).
- Source Concepts from place of service vocabularies are mapped into these standard visit Concepts in the Standardized Vocabularies.
- At any one day, there could be more than one visit.
- One visit may involve multiple providers, in which case the ETL must specify how a single provider id is selected or leave the provider_id field null.
- One visit may involve multiple Care Sites, in which case the ETL must specify how a single care_site id is selected or leave the care_site_id field null.
- Visits are recorded in various data sources in different forms with varying levels of standardization. For example:
- Medical Claims include Inpatient Admissions, Outpatient Services, and Emergency Room visits.
- Electronic Health Records may capture Person visits as part of the activities recorded depending wether the EHR system is used at the different Care Sites.
- Sequential relationships between Visits within an episode of care are represented through chaining them in the preceding_visit_occurrence_id.
PROCEDURE_OCCURRENCE table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table changed in version 5.1 of the OMOP CDM. The field procedure_datetime was added.
The PROCEDURE_OCCURRENCE tabe contains records of activities or processes ordered by, or carried out by, a healthcare provider on the patient to have a diagnostic or therapeutic purpose. Procedures are present in various data sources in different forms with varying levels of standardization. For example:
- Medical Claims include procedure codes that are submitted as part of a claim for health services rendered, including procedures performed.
- Electronic Health Records that capture procedures as orders.
| Field | Required | Type | Description |
|---|---|---|---|
| procedure_occurrence_id | Yes | integer | A system-generated unique identifier for each Procedure Occurrence. |
| person_id | Yes | integer | A foreign key identifier to the Person who is subjected to the Procedure. The demographic details of that Person are stored in the PERSON table. |
| procedure_concept_id | Yes | integer | A foreign key that refers to a standard procedure Concept identifier in the Standardized Vocabularies. |
| procedure_date | Yes | date | The date on which the Procedure was performed. |
| procedure_datetime | No | datetime | The date and time on which the Procedure was performed. |
| procedure_type_concept_id | Yes | integer | A foreign key to the predefined Concept identifier in the Standardized Vocabularies reflecting the type of source data from which the procedure record is derived. |
| modifier_concept_id | No | integer | A foreign key to a Standard Concept identifier for a modifier to the Procedure (e.g. bilateral) |
| quantity | No | integer | The quantity of procedures ordered or administered. |
| provider_id | No | integer | A foreign key to the provider in the provider table who was responsible for carrying out the procedure. |
| visit_occurrence_id | No | integer | A foreign key to the visit in the visit table during which the Procedure was carried out. |
| procedure_source_value | No | varchar(50) | The source code for the Procedure as it appears in the source data. This code is mapped to a standard procedure Concept in the Standardized Vocabularies and the original code is, stored here for reference. Procedure source codes are typically ICD-9-Proc, CPT-4, HCPCS or OPCS-4 codes. |
| procedure_source_concept_id | No | integer | A foreign key to a Procedure Concept that refers to the code used in the source. |
| qualifier_source_value | No | varchar(50) | The source code for the qualifier as it appears in the source data. |
Conventions
- Valid Procedure Concepts belong to the “Procedure” domain. Procedure Concepts are based on a variety of vocabularies: SNOMED-CT, ICD-9-Proc, CPT-4, HCPCS and OPCS-4, but also atypical Vocabularies such as ICD-9-CM or MedDRA.
- Procedures are expected to be carried out within one day and therefore have no end date.
- Procedures could involve the application of a drug, in which case the procedural component is recorded in the procedure table and simultaneously the administered drug in the drug exposure table when both the procedural component and drug are identifiable.
- If the quantity value is omitted, a single procedure is assumed.
- The Procedure Type defines from where the Procedure Occurrence is drawn or inferred. For administrative claims records the type indicates whether a Procedure was primary or secondary and their relative positioning within a claim.
- The Visit during which the procedure was performed is recorded through a reference to the VISIT_OCCURRENCE table. This information is not always available.
- The Provider carrying out the procedure is recorded through a reference to the PROVIDER table. This information is not always available.
DRUG_EXPOSURE table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table changed in version 5.1 of the OMOP CDM. The fields drug_exposure_start_datetime and drug_exposure_end_datetime were added.
The drug exposure domain captures records about the utilization of a Drug when ingested or otherwise introduced into the body. A Drug is a biochemical substance formulated in such a way that when administered to a Person it will exert a certain physiological effect. Drugs include prescription and over-the-counter medicines, vaccines, and large-molecule biologic therapies. Radiological devices ingested or applied locally do not count as Drugs.
Drug Exposure is inferred from clinical events associated with orders, prescriptions written, pharmacy dispensings, procedural administrations, and other patient-reported information, for example:
- The “Prescription” section of an EHR captures prescriptions written by physicians or from electronic ordering systems
- The “Medication list” section of an EHR for both non-prescription products and medications prescribed by other providers
- Prescriptions filled at dispensing providers such as pharmacies, and then captured in reimbursement claim systems
- Drugs administered as part of a Procedure, such as chemotherapy or vaccines.
| Field | Required | Type | Description |
|---|---|---|---|
| drug_exposure_id | Yes | integer | A system-generated unique identifier for each Drug utilization event. |
| person_id | Yes | integer | A foreign key identifier to the person who is subjected to the Drug. The demographic details of that person are stored in the person table. |
| drug_concept_id | Yes | integer | A foreign key that refers to a Standard Concept identifier in the Standardized Vocabularies for the Drug concept. |
| drug_exposure_start_date | Yes | date | The start date for the current instance of Drug utilization. Valid entries include a start date of a prescription, the date a prescription was filled, or the date on which a Drug administration procedure was recorded. |
| drug_exposure_start_datetime | No | datetime | The start date and time for the current instance of Drug utilization. Valid entries include a start date of a prescription, the date a prescription was filled, or the date on which a Drug administration procedure was recorded. |
| drug_exposure_end_date | No | date | The end date for the current instance of Drug utilization. It is not available from all sources. |
| drug_exposure_end_datetime | No | datetime | The end date and time for the current instance of Drug utilization. It is not available from all sources. |
| drug_type_concept_id | Yes | integer | A foreign key to the predefined Concept identifier in the Standardized Vocabularies reflecting the type of Drug Exposure recorded. It indicates how the Drug Exposure was represented in the source data. |
| stop_reason | No | varchar(20) | The reason the Drug was stopped. Reasons include regimen completed, changed, removed, etc. |
| refills | No | integer | The number of refills after the initial prescription. The initial prescription is not counted, values start with 0. |
| quantity | No | float | The quantity of drug as recorded in the original prescription or dispensing record. |
| days_supply | No | integer | The number of days of supply of the medication as recorded in the original prescription or dispensing record. |
| sig | No | clob | The directions (“signetur”) on the Drug prescription as recorded in the original prescription (and printed on the container) or dispensing record. |
| route_concept_id | No | integer | A foreign key to a predefined concept in the Standardized Vocabularies reflecting the route of administration. |
| effective_drug_dose | No | float | Numerical value of Drug dose for this Drug Exposure record. |
| dose_unit_concept_ id | No | integer | A foreign key to a predefined concept in the Standardized Vocabularies reflecting the unit the effective_drug_dose value is expressed. |
| lot_number | No | varchar(50) | An identifier assigned to a particular quantity or lot of Drug product from the manufacturer. |
| provider_id | No | integer | A foreign key to the provider in the provider table who initiated (prescribed or administered) the Drug Exposure. |
| visit_occurrence_id | No | integer | A foreign key to the visit in the visit table during which the Drug Exposure was initiated. |
| drug_source_value | No | varchar(50) | The source code for the Drug as it appears in the source data. This code is mapped to a Standard Drug concept in the Standardized Vocabularies and the original code is, stored here for reference. |
| drug_source_concept_id | No | integer | A foreign key to a Drug Concept that refers to the code used in the source. |
| route_source_value | No | varchar(50) | The information about the route of administration as detailed in the source. |
| dose_unit_source_value | No | varchar(50) | The information about the dose unit as detailed in the source. |
Conventions
- Valid Concepts for the drug_concept_id field belong to the “Drug” domain. Most Concepts in the Drug domain are based on RxNorm, but some may come from other sources. Concepts are members of the Clinical Drug or Pack, Branded Drug or Pack, Drug Component or Ingredient classes.
- Source drug identifiers, including NDC codes, Generic Product Identifiers, etc. are mapped to Standard Drug Concepts in the Standardized Vocabularies (e.g., based on RxNorm). When the Drug Source Value of the code cannot be translated into standard Drug Concept IDs, a Drug exposure entry is stored with only the corresponding source_concept_id and drug_source_value and a drug_concept_id of 0.
- The Drug Concept with the most detailed content of information is preferred during the mapping process. These are indicated in the concept_class_id field of the Concept and are recorded in the following order of precedence: “Marketed Product”, “Branded Pack”, “Clinical Pack”, “Branded Drug”, “Clinical Drug”, “Branded Drug Component”, “Clinical Drug Component”, “Branded Drug Form”, “Clinical Drug Form”, and only if no other information is available “Ingredient”. Note: If only the drug class (i.e. “Diuretic”, “NSAID”) is known, the drug_concept_id should contain 0.
- A Drug Type is assigned to each Drug Exposure to track from what source the information was drawn or inferred from. The valid domain_id for these Concepts is “Drug Type”.
- The content of the refills field determines the current number of refills, not the number of remaining refills. For example, for a drug prescription with 2 refills, the content of this field for the 3 Drug Exposure events are null, 1 and 2.
- The route_concept_id refers to a Standard Concepts of the “Route” domain. Note: Route information can also be inferred from the Drug product itself by determining the Drug Form of the Concept, creating some partial overlap of the same type of information. However, the route_concept_id could resolve ambiguities of how a certain Drug Form is actually applied. For example, a “Solution” could be used orally or parentherally, and this field will make this determination.
- The Effective Drug Dose and the Dose Unit Concepts are provided in cases when dose information is explicitly provided, as it is typically for pediatric and chemotherapeutic treatments. The domain_id for the Dose Unit Concept is “Unit”. Note: this information can only be present if the Drug contains a single active ingredient. Combination products which have doses for each ingredient need to be recorded as separate records.
- The lot_number field contains an identifier assigned from the manufacturer of the Drug product.
- If possible, the visit in which the drug was prescribed or delivered is recorded in the visit_occurrence_id field through a reference to the visit table.
- If possible, the prescribing or administering provider (physician or nurse) is recorded in the provider_id field through a reference to the provider table.
DEVICE_EXPOSURE table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table changed in version 5.1 of the OMOP CDM. The fields device_exposure_start_datetime and device_exposure_end_datetime were added.
The device exposure domain captures information about a person’s exposure to a foreign physical object or instrument that which is used for diagnostic or therapeutic purposes through a mechanism beyond chemical action. Devices include implantable objects (e.g. pacemakers, stents, artificial joints), medical equipment and supplies (e.g. bandages, crutches, syringes), other instruments used in medical procedures (e.g. sutures, defibrillators) and material used in clinical care (e.g. adhesives, body material, dental material, surgical material).
| Field | Required | Type | Description |
|---|---|---|---|
| device_exposure_id | Yes | integer | A system-generated unique identifier for each Device Exposure. |
| person_id | Yes | integer | A foreign key identifier to the Person who is subjected to the Device. The demographic details of that person are stored in the Person table. |
| device_concept_id | Yes | integer | A foreign key that refers to a Standard Concept identifier in the Standardized Vocabularies for the Device concept. |
| device_exposure_start_date | Yes | date | The date the Device or supply was applied or used. |
| device_exposure_start_datetime | No | datetime | The date and time the Device or supply was applied or used. |
| device_exposure_end_date | No | date | The date the Device or supply was removed from use. |
| device_exposure_end_datetime | No | datetime | The date and time the Device or supply was removed from use. |
| device_type_concept_id | Yes | integer | A foreign key to the predefined Concept identifier in the Standardized Vocabularies reflecting the type of Device Exposure recorded. It indicates how the Device Exposure was represented in the source data. |
| unique_device_id | No | varchar(50) | A UDI or equivalent identifying the instance of the Device used in the Person. |
| quantity | No | integer | The number of individual Devices used for the exposure. |
| provider_id | No | integer | A foreign key to the provider in the PROVIDER table who initiated of administered the Device. |
| visit_occurrence_id | No | integer | A foreign key to the visit in the VISIT table during which the device was used. |
| device_source_value | No | varchar(50) | The source code for the Device as it appears in the source data. This code is mapped to a standard Device Concept in the Standardized Vocabularies and the original code is stored here for reference. |
| device_source_ concept_id | No | integer | A foreign key to a Device Concept that refers to the code used in the source. |
Conventions
- The distinction between Devices or supplies and procedures are sometimes blurry, but the former are physical objects while the latter are actions, often to apply a Device or supply.
- For medical devices that are regulated by the FDA, if a Unique Device Identification (UDI) is provided if available in the data source, and is recorded in the unique_device_id field.
- Valid Device Concepts belong to the “Device” domain. The Concepts of this domain are derived from the DI portion of a UDI or based on other source vocabularies, like HCPCS.
- A Device Type is assigned to each Device Exposure to track from what source the information was drawn or inferred. The valid domain_id for these Concepts is “Device Type”.
- The Visit during which the Device was first used is recorded through a reference to the VISIT_OCCURRENCE table. This information is not always available.
- The Provider exposing the patient to the Device is recorded through a reference to the PROVIDER table. This information is not always available.
CONDITION_OCCURRENCE table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table changed in version 5.1 of the OMOP CDM. The fields condition_start_datetime and condition_end_datetime were added. For 5.0.1, this table changed in version 5.X of the OMOP CDM. The fields condition_status_concept_id and condition_status_source_value were added.
Conditions are records of a Person suggesting the presence of a disease or medical condition stated as a diagnosis, a sign or a symptom, which is either observed by a Provider or reported by the patient. Conditions are recorded in different sources and levels of standardization, for example:
- Medical claims data include diagnoses coded in ICD-9-CM that are submitted as part of a reimbursement claim for health services and
- EHRs may capture Person Conditions in the form of diagnosis codes or symptoms.
| Field | Required | Type | Description |
|---|---|---|---|
| condition_occurrence_id | Yes | integer | A unique identifier for each Condition Occurrence event. |
| person_id | Yes | integer | A foreign key identifier to the Person who is experiencing the condition. The demographic details of that Person are stored in the PERSON table. |
| condition_concept_id | Yes | integer | A foreign key that refers to a Standard Condition Concept identifier in the Standardized Vocabularies. |
| condition_start_date | Yes | date | The date when the instance of the Condition is recorded. |
| condition_start_datetime | No | datetime | The date and time when the instance of the Condition is recorded. |
| condition_end_date | No | date | The date when the instance of the Condition is considered to have ended. |
| condition_end_datetime | No | date | The date when the instance of the Condition is considered to have ended. |
| condition_type_concept_id | Yes | integer | A foreign key to the predefined Concept identifier in the Standardized Vocabularies reflecting the source data from which the condition was recorded, the level of standardization, and the type of occurrence. |
| stop_reason | No | varchar(20) | The reason that the condition was no longer present, as indicated in the source data. |
| provider_id | No | integer | A foreign key to the Provider in the PROVIDER table who was responsible for capturing (diagnosing) the Condition. |
| visit_occurrence_id | No | integer | A foreign key to the visit in the VISIT table during which the Condition was determined (diagnosed). |
| condition_status_concept_id | No | integer | A foreign key to the predefined concept in the standard vocabulary reflecting the condition status. |
| condition_source_concept_id | No | integer | A foreign key to a Condition Concept that refers to the code used in the source. |
| condition_source_value | No | varchar(50) | The source code for the condition as it appears in the source data. This code is mapped to a standard condition concept in the Standardized Vocabularies and the original code is stored here for reference. |
| condition_status_source_value | No | varchar(50) |
Conventions
- Valid Condition Concepts belong to the “Condition” domain.
- Condition records are typically inferred from diagnostic codes recorded in the source data. Such code system, like ICD-9-CM, ICD-10-CM, Read etc., provide a comprehensive coverage of conditions. However, if the diagnostic code in the source does not define a condition, but rather an observation or a procedure, then such information is not stored in the CONDITION_OCCURRENCE table, but in the respective tables instead.
- Source Condition identifiers are mapped to Standard Concepts for Conditions in the Standardized Vocabularies. When the source code cannot be translated into a Standard Concept, a CONDITION_OCCURRENCE entry is stored with only the corresponding source_concept_id and source_value, while the condition_concept_id is set to 0.
- Family history and past diagnoses (“history of”) are not recorded in the CONDITION_OCCURRENCE table. Instead, they are listed in the OBSERVATION table.
- Codes written in the process of establishing the diagnosis, such as “question of” of and “rule out”, are not represented here. Instead, they are listed in the OBSERVATION table, if they are used for analyses.
- A Condition Occurrence Type is assigned based on the data source and type of condition attribute, for example:
- ICD-9-CM Primary Diagnosis from inpatient and outpatient Claims
- ICD-9-CM Secondary Diagnoses from inpatient and outpatient Claims
- Diagnoses or problems recorded in an EHR.
- The Stop Reason indicates why a Condition is no longer valid with respect to the purpose within the source data. Typical values include “Discharged”, “Resolved”, etc. Note that a Stop Reason does not necessarily imply that the condition is no longer occurring.
- Condition source codes are typically ICD-9-CM, Read or ICD-10 diagnosis codes from medical claims or discharge status/visit diagnosis codes from EHRs.
- The Condition Status reflects when the condition was diagnosed, implying a different depth of diagnostic work-up:
- Admitting diagnosis: use concept_id 4203942
- Preliminary diagnosis: use concept_id 4033240
- Final diagnosis: use concept_id 4230359 – should also be used for ‘Discharge diagnosis’
MEASUREMENT table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table changed in version 5.1 of the OMOP CDM. The field measurement_datetime was added.
The MEASUREMENT table contains records of Measurement, i.e. structured values (numerical or categorical) obtained through systematic and standardized examination or testing of a Person or Person's sample. The MEASUREMENT table contains both orders and results of such Measurements as laboratory tests, vital signs, quantitative findings from pathology reports, etc.
| Field | Required | Type | Description |
|---|---|---|---|
| measurement_id | Yes | integer | A unique identifier for each Measurement. |
| person_id | Yes | integer | A foreign key identifier to the Person about whom the measurement was recorded. The demographic details of that Person are stored in the PERSON table. |
| measurement_concept_id | Yes | integer | A foreign key to the standard measurement concept identifier in the Standardized Vocabularies. |
| measurement_date | Yes | date | The date of the Measurement. |
| measurement_datetime | No | datetime | The date and time of the Measurement. (Some database systems don't have a datatype of time. To accomodate all temporal analyses, datatype datetime can be used (combining measurement_date and measurement_time)Relevant Forum Discussion |
| measurement_type_concept_id | Yes | integer | A foreign key to the predefined Concept in the Standardized Vocabularies reflecting the provenance from where the Measurement record was recorded. |
| operator_concept_id | No | integer | A foreign key identifier to the predefined Concept in the Standardized Vocabularies reflecting the mathematical operator that is applied to the value_as_number. Operators are <, ≤, =, ≥, >. |
| value_as_number | No | float | A Measurement result where the result is expressed as a numeric value. |
| value_as_concept_id | No | integer | A foreign key to a Measurement result represented as a Concept from the Standardized Vocabularies (e.g., positive/negative, present/absent, low/high, etc.). |
| unit_concept_id | No | integer | A foreign key to a Standard Concept ID of Measurement Units in the Standardized Vocabularies. |
| range_low | No | float | The lower limit of the normal range of the Measurement result. The lower range is assumed to be of the same unit of measure as the Measurement value. |
| range_high | No | float | The upper limit of the normal range of the Measurement. The upper range is assumed to be of the same unit of measure as the Measurement value. |
| provider_id | No | integer | A foreign key to the provider in the PROVIDER table who was responsible for initiating or obtaining the measurement. |
| visit_occurrence_id | No | integer | A foreign key to the Visit in the VISIT_OCCURRENCE table during which the Measurement was recorded. |
| measurement_source_value | No | varchar(50) | The Measurement name as it appears in the source data. This code is mapped to a Standard Concept in the Standardized Vocabularies and the original code is stored here for reference. |
| measurement_source_concept_id | No | integer | A foreign key to a Concept in the Standard Vocabularies that refers to the code used in the source. |
| unit_source_value | No | varchar(50) | The source code for the unit as it appears in the source data. This code is mapped to a standard unit concept in the Standardized Vocabularies and the original code is stored here for reference. |
| value_source_value | No | varchar(50) | The source value associated with the content of the value_as_number or value_as_concept_id as stored in the source data. |
Conventions
- Measurements differ from Observations in that they require a standardized test or some other activity to generate a quantitative or qualitative result. For example, LOINC 1755-8 concept_id 3027035 'Albumin [Mass/time] in 24 hour Urine' is the lab test to measure a certain chemical in a urine sample.
- Even though each Measurement always have a result, the fields value_as_number and value_as_concept_id are not mandatory. When the result is not known, the Measurement record represents just the fact that the corresponding Measurement was carried out, which in itself is already useful information for some use cases.
- Valid Measurement Concepts (measurement_concept_id) belong to the 'Measurement' domain, but could overlap with the 'Observation' domain. This is due to the fact that there is a continuum between systematic examination or testing (Measurement) and a simple determination of fact (Observation). When the Measurement Source Value of the code cannot be translated into a standard Measurement Concept ID, a Measurement entry is stored with only the corresponding source_concept_id and measurement_source_value and a measurement_concept_id of 0.
- Measurements are stored as attribute value pairs, with the attribute as the Measurement Concept and the value representing the result. The value can be a Concept (stored in value_as_concept), or a numerical value (value_as_number) with a Unit (unit_concept_id).
- Valid Concepts for the value_as_concept field belong to the 'Meas Value' domain.
- For some Measurement Concepts, the result is included in the test. For example, ICD10 concept_id 45595451 “Presence of alcohol in blood, level not specified” indicates a Measurement and the result (present). In those situations, the CONCEPT_RELATIONSHIP table in addition to the “Maps to” record contains a second record with the relationship_id set to “Maps to value”. In this example, the “Maps to” relationship directs to 4041715 “Blood ethanol measurement” as well as a “Maps to value” record to 4181412 “Present”.
- The operator_concept_id is optionally given for relative Measurements where the precise value is not available but its relation to a certain benchmarking value is. For example, this can be used for minimal detection thresholds of a test.
- The meaning of Concept 4172703 for '=' is identical to omission of a operator_concept_id value. Since the use of this field is rare, it's important when devising analyses to not to forget testing for the content of this field for values different from =.
- Valid Concepts for the operator_concept_id field belong to the 'Meas Value Operator' domain.
- The Unit is optional even if a value_as_number is provided.
- If reference ranges for upper and lower limit of normal as provided (typically by a laboratory) these are stored in the range_high and range_low fields. Ranges have the same unit as the value_as_number.
- The Visit during which the observation was made is recorded through a reference to the VISIT_OCCURRENCE table. This information is not always available.
- The Provider making the observation is recorded through a reference to the PROVIDER table. This information is not always available.
NOTE table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table changed in version 5.2 of the OMOP CDM. The fields note_datetime, note_class_concept_id, note_title, encoding_concept_id, language_concept_id were added.
The NOTE table captures unstructured information that was recorded by a provider about a patient in free text notes on a given date.
| Field | Required | Type | Description |
|---|---|---|---|
| note_id | Yes | integer | A unique identifier for each note. |
| person_id | Yes | integer | A foreign key identifier to the Person about whom the Note was recorded. The demographic details of that Person are stored in the PERSON table. |
| note_date | Yes | date | The date the note was recorded. |
| note_datetime | No | datetime | The date and time the note was recorded. |
| note_type_concept_id | Yes | integer | A foreign key to the predefined Concept in the Standardized Vocabularies reflecting the type, origin or provenance of the Note. |
| note_class_concept_id | Yes | integer | A foreign key to the predefined Concept in the Standardized Vocabularies reflecting the HL7 LOINC Document Type Vocabulary classification of the note. |
| note_title | No | string(250) | The title of the Note as it appears in the source. |
| note_text | No | RBDMS dependent text | The content of the Note. |
| encoding_concept_id | Yes | integer | A foreign key to the predefined Concept in the Standardized Vocabularies reflecting the note character encoding type. |
| language_concept_id | Yes | integer | A foreign key to the predefined Concept in the Standardized Vocabularies reflecting the language of the note. |
| provider_id | No | integer | A foreign key to the Provider in the PROVIDER table who took the Note. |
| visit_occurrence_id | No | integer | Foreign key to the Visit in the VISIT_OCCURRENCE table when the Note was taken. |
Conventions
- The NOTE table contains free text (in ASCII, or preferably in UTF8 format) taken by a healthcare Provider.
- The Visit during which the note was written is recorded through a reference to the VISIT_OCCURRENCE table. This information is not always available.
- The Provider making the note is recorded through a reference to the PROVIDER table. This information is not always available.
- The type of note_text is CLOB or string(MAX) depending on RDBMS
OBSERVATION table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
This table changed in version 5.1 of the OMOP CDM. The field observation_datetime was added.
The OBSERVATION table captures clinical facts about a Person obtained in the context of examination, questioning or a procedure. Any data that cannot be represented by any other domains, such as social and lifestyle facts, medical history, family history, etc. are recorded here.
| Field | Required | Type | Description |
|---|---|---|---|
| observation_id | Yes | integer | A unique identifier for each observation. |
| person_id | Yes | integer | A foreign key identifier to the Person about whom the observation was recorded. The demographic details of that Person are stored in the PERSON table. |
| observation_concept_id | Yes | integer | A foreign key to the standard observation concept identifier in the Standardized Vocabularies. |
| observation_date | Yes | date | The date of the observation. |
| observation_datetime | No | datetime | The date and time of the observation. |
| observation_type_concept_id | Yes | integer | A foreign key to the predefined concept identifier in the Standardized Vocabularies reflecting the type of the observation. |
| value_as_number | No | float | The observation result stored as a number. This is applicable to observations where the result is expressed as a numeric value. |
| value_as_string | No | varchar(60) | The observation result stored as a string. This is applicable to observations where the result is expressed as verbatim text. |
| value_as_concept_id | No | Integer | A foreign key to an observation result stored as a Concept ID. This is applicable to observations where the result can be expressed as a Standard Concept from the Standardized Vocabularies (e.g., positive/negative, present/absent, low/high, etc.). |
| qualifier_concept_id | No | integer | A foreign key to a Standard Concept ID for a qualifier (e.g., severity of drug-drug interaction alert) |
| unit_concept_id | No | integer | A foreign key to a Standard Concept ID of measurement units in the Standardized Vocabularies. |
| provider_id | No | integer | A foreign key to the provider in the PROVIDER table who was responsible for making the observation. |
| visit_occurrence_id | No | integer | A foreign key to the visit in the VISIT_OCCURRENCE table during which the observation was recorded. |
| observation_source_value | No | varchar(50) | The observation code as it appears in the source data. This code is mapped to a Standard Concept in the Standardized Vocabularies and the original code is, stored here for reference. |
| observation_source_concept_id | No | integer | A foreign key to a Concept that refers to the code used in the source. |
| unit_source_value | No | varchar(50) | The source code for the unit as it appears in the source data. This code is mapped to a standard unit concept in the Standardized Vocabularies and the original code is, stored here for reference. |
| qualifier_source_value | No | varchar(50) | The source value associated with a qualifier to characterize the observation |
Conventions
- Observations differ from Measurements in that they do not require a standardized test or some other activity to generate clinical fact. Typical observations are medical history, family history, the stated need for certain treatment, social circumstances, lifestyle choices, healthcare utilization patterns, etc. If the generation clinical facts requires a standardized testing such as lab testing or imaging and leads to a standardized result, the data item is recorded in the MEASUREMENT table. If the clinical fact observed determines a sign, symptom, diagnosis of a disease or other medical condition, it is recorded in the CONDITION_OCCURRENCE table.
- Valid Observation Concepts are not enforced to be from any domain. They still should be Standard Concepts, and they typically belong to the “Observation” or sometimes “Measurement” domain.
- Observation can be stored as attribute value pairs, with the attribute as the Observation Concept and the value representing the clinical fact. This fact can be a Concept (stored in value_as_concept), a numerical value (value_as_number) or a verbatim string (value_as_string). Even though Observations do not have an explicit result, the clinical fact can be stated separately from the type of Observation in the value_as_ fields.
- It is recommended for observations that are suggestive statements of positive assertion should have a value of “Yes” (concept_id=4188539), recorded, even though the null value is the equivalent.
- Valid Concepts of the value_as_concept field are not enforced, but typically belong to the “Meas Value” domain.
- For numerical facts a Unit can be provided in the unit_concept_id.
- For facts represented as Concepts no domain membership is enforced.
- Note that the value of value_as_concept_id may be provided through mapping from a source Concept which contains the content of the Observation. In those situations, the CONCEPT_RELATIONSHIP table in addition to the “Maps to” record contains a second record with the relationship_id set to “Maps to value”. For example, ICD9CM V17.5 concept_id 44828510 “Family history of asthma” has a “Maps to” relationship to 4167217 “Family history of clinical finding” as well as a “Maps to value” record to 317009 “Asthma”.
- The qualifier_concept_id field contains all attributes specifying the clinical fact further, such as as degrees, severities, drug-drug interaction alerts etc.
- The Visit during which the observation was made is recorded through a reference to the VISIT_OCCURRENCE table. This information is not always available.
- The Provider making the observation is recorded through a reference to the PROVIDER table. This information is not always available.
FACT_RELATIONSHIP table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The FACT_RELATIONSHIP table contains records about the relationships between facts stored as records in any table of the CDM. Relationships can be defined between facts from the same domain (table), or different domains. Examples of Fact Relationships include: Person relationships (parent-child), care site relationships (hierarchical organizational structure of facilities within a health system), indication relationship (between drug exposures and associated conditions), usage relationships (of devices during the course of an associated procedure), or facts derived from one another (measurements derived from an associated specimen).
| Field | Required | Type | Description |
|---|---|---|---|
| domain_concept _id_1 | Yes | integer | The concept representing the domain of fact one, from which the corresponding table can be inferred. |
| fact_id_1 | Yes | integer | The unique identifier in the table corresponding to the domain of fact one. |
| domain_concept_id_2 | Yes | integer | The concept representing the domain of fact two, from which the corresponding table can be inferred. |
| fact_id_2 | Yes | integer | The unique identifier in the table corresponding to the domain of fact two. |
| relationship_concept_id | Yes | integer | A foreign key to a Standard Concept ID of relationship in the Standardized Vocabularies. |
Conventions
- All relationships are directional, and each relationship is represented twice symmetrically within the FACT_RELATIONSHIP table. For example, two persons if person_id = 1 is the mother of person_id = 2 two records are in the FACT_RELATIONSHIP table (all strings in fact concept_id records in the Concept table:
- Person, 1, Person, 2, parent of
- Person, 2, Person, 1, child of
Standardized Health System Data Tables
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
These tables describe the healthcare provider system responsible for administering the healthcare of the patient, rather than the demographic or clinical events the patient experienced.
Below provides an entity-relationship diagram highlighting the tables within the Standardized Health System portion of the OMOP Common Data Model:
Standardized Health System Data Entity-Relationship diagram
LOCATION table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The LOCATION table represents a generic way to capture physical location or address informationof Persons and Care Sites.
| Field | Required | Type | Description |
|---|---|---|---|
| location_id | Yes | integer | A unique identifier for each geographic location. |
| address_1 | No | varchar(50) | The address field 1, typically used for the street address, as it appears in the source data. |
| address_2 | No | varchar(50) | The address field 2, typically used for additional detail such as buildings, suites, floors, as it appears in the source data. |
| city | No | varchar(50) | The city field as it appears in the source data. |
| state | No | varchar(2) | The state field as it appears in the source data. |
| zip | No | varchar(9) | The zip or postal code. |
| county | No | varchar(20) | The county. |
| location_source_value | No | varchar(50) | The verbatim information that is used to uniquely identify the location as it appears in the source data. |
Conventions
- Each address or Location is unique and is present only once in the table.
- Locations do not contain names, such as the name of a hospital. In order to construct a full address that can be used in the postal service, the address information from the Location needs to be combined with information from the Care Site. The PERSON table does not contain name information at all.
- All fields in the Location tables contain the verbatim data in the source, no mapping or normalization takes place. None of the fields are mandatory. If the source data have no Location information at all, all Locations are represented by a single record. Typically, source data contain full or partial zip or postal codes or county or census district information.
- Zip codes are handled as strings of up to 9 characters length. For US addresses, these represent either a 3-digit abbreviated Zip code as provided by many sources for patient protection reasons, the full 5-digit Zip or the 9-digit (ZIP + 4) codes. Unless for specific reasons analytical methods should expect and utilize only the first 3 digits. For international addresses, different rules apply.
- The county information can be provided and is not redundant with information from the zip codes as not all of these have an unambiguous county designation.
- No country information is expected as source data are always collected within a single country.
CARE_SITE table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The CARE_SITE table contains a list of uniquely identified institutional (physical or organizational) units where healthcare delivery is practiced (offices, wards, hospitals, clinics, etc.).
| Field | Required | Type | Description |
|---|---|---|---|
| care_site_id | Yes | integer | A unique identifier for each Care Site. |
| care_site_name | No | varchar(255) | The verbatim description or name of the Care Site as in data source |
| place_of_service_concept_id | No | integer | A foreign key that refers to a Place of Service Concept ID in the Standardized Vocabularies. |
| location_id | No | integer | A foreign key to the geographic Location in the LOCATION table, where the detailed address information is stored. |
| care_site_source_value | No | varchar(50) | The identifier for the Care Site in the source data, stored here for reference. |
| place_of_service_source_value | No | varchar(50) | The source code for the Place of Service as it appears in the source data, stored here for reference. |
Conventions
- Care site is a unique combination of location_id and place_of_service_source_value.
- Every record in the visit_occurrence table may have only one care site
- Care site does not take into account the provider (human) information such a specialty.
- Many source data do not make a distinction between individual and institutional providers. The CARE_SITE table contains the institutional providers.
- If the source, instead of uniquely identifying individual Care Sites, only provides limited information such as Place of Service, generic or “pooled” Care Site records are listed in the CARE_SITE table.
- There are hierarchical and business relationships between Care Sites. For example,wards can belong to clinics or departments, which can in turn belong to hospitals, which in turn can belong to hospital systems, which in turn can belong to HMOs.
- The relationships between Care Sites are defined in the FACT_RELATIONSHIP table.
- The Care Site Source Value typically contains the name of the Care Site.
- The Place of Service Concepts belongs to the Domain 'Place of Service'.
PROVIDER table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The PROVIDER table contains a list of uniquely identified healthcare providers. These are individuals providing hands-on healthcare to patients, such as physicians, nurses, midwives, physical therapists etc.
| Field | Required | Type | Description |
|---|---|---|---|
| provider_id | Yes | integer | A unique identifier for each Provider. |
| provider_name | No | varchar(50) | A description of the Provider. |
| npi | No | varchar(20) | The National Provider Identifier (NPI) of the provider. |
| dea | No | varchar(20) | The Drug Enforcement Administration (DEA) number of the provider. |
| specialty_concept_id | No | integer | A foreign key to a Standard Specialty Concept ID in the Standardized Vocabularies. |
| care_site_id | No | integer | A foreign key to the main Care Site where the provider is practicing. |
| year_of_birth | No | integer | The year of birth of the Provider. |
| gender_concept_id | No | integer | The gender of the Provider. |
| provider_source_value | No | varchar(50) | The identifier used for the Provider in the source data, stored here for reference. |
| specialty_source_value | No | varchar(50) | The source code for the Provider specialty as it appears in the source data, stored here for reference. |
| specialty_source_concept_id | No | integer | A foreign key to a Concept that refers to the code used in the source. |
| gender_source_value | No | varchar(50) | The gender code for the Provider as it appears in the source data, stored here for reference. |
| gender_source_concept_id | No | integer | A foreign key to a Concept that refers to the code used in the source. |
Conventions
- Many sources do not make a distinction between individual and institutional providers. The PROVIDER table contains the individual providers.
- If the source, instead of uniquely identifying individual providers, only provides limited information such as specialty, generic or “pooled” Provider records are listed in the PROVIDER table.
- A single Provider cannot be listed twice (be duplicated) in the table. If a Provider has more than one Specialty, the main or most often exerted specialty should be recorded.
- Valid Specialty Concepts belong to the 'Specialty' domain.
- The care_site_id represent a fixed relationship between a Provider and her main Care Site. Providers are also linked to Care Sites through Condition, Procedure and Visit records.
Standardized Health Economics Data Tables
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
These tables contain cost information about healthcare. They are dependent on the healthcare delivery system the patient is involved in, which may vary significantly within a country and across different countries. However, the current model is focused on the US healthcare system.
Below provides an entity-relationship diagram highlighting the tables within the Standardized Health Economics portion of the OMOP Common Data Model:
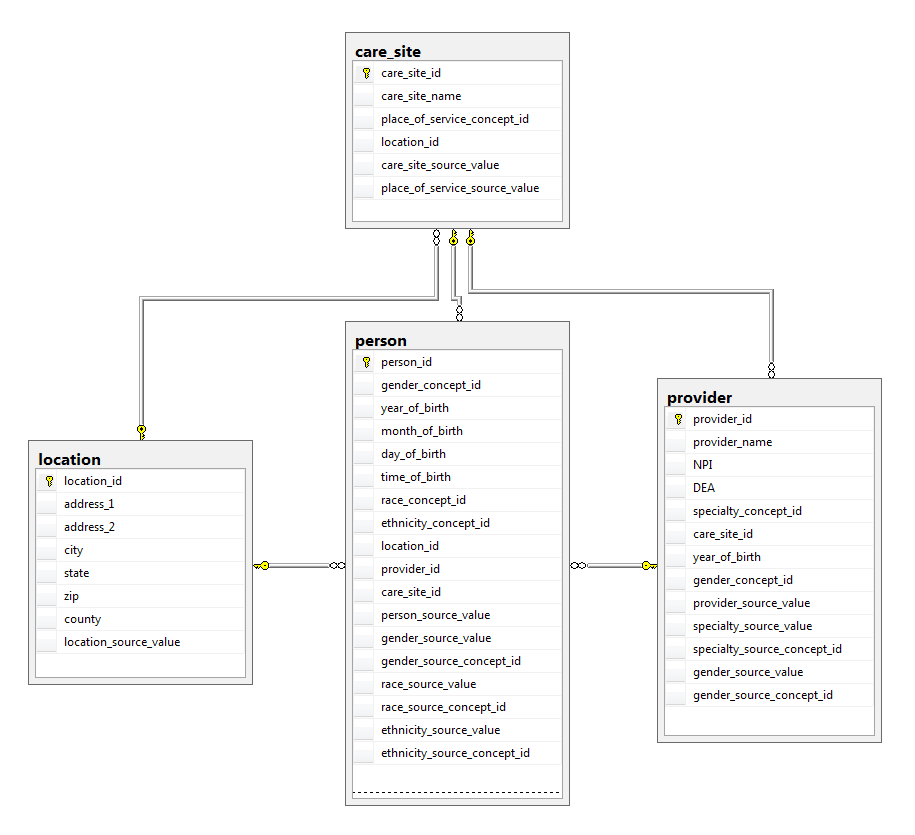 Standardized Health Economics Data Entity-Relationship diagram
Standardized Health Economics Data Entity-Relationship diagram
PAYER_PLAN_PERIOD table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The PAYER_PLAN_PERIOD table captures details of the period of time that a Person is continuously enrolled under a specific health Plan benefit structure from a given Payer. Each Person receiving healthcare is typically covered by a health benefit plan, which pays for (fully or partially), or directly provides, the care. These benefit plans are provided by payers, such as health insurances or state or government agencies. In each plan the details of the health benefits are defined for the Person or her family, and the health benefit Plan might change over time typically with increasing utilization (reaching certain cost thresholds such as deductibles), plan availability and purchasing choices of the Person. The unique combinations of Payer organizations, health benefit Plans and time periods in which they are valid for a Person are recorded in this table.
| Field | Required | Type | Description |
|---|---|---|---|
| payer_plan_period_id | Yes | integer | A identifier for each unique combination of payer, plan, family code and time span. |
| person_id | Yes | integer | A foreign key identifier to the Person covered by the payer. The demographic details of that Person are stored in the PERSON table. |
| payer_plan_period_start_date | Yes | date | The start date of the payer plan period. |
| payer_plan_period_end_date | Yes | date | The end date of the payer plan period. |
| payer_source_value | No | varchar(50) | The source code for the payer as it appears in the source data. |
| plan_source_value | No | varchar(50) | The source code for the Person's health benefit plan as it appears in the source data. |
| family_source_value | No | varchar(50) | The source code for the Person's family as it appears in the source data. |
Conventions
- Different Payers have different designs for their health benefit Plans. The PAYER_PLAN_PERIOD table does not capture all details of the plan design or the relationship between Plans or the cost of healthcare triggering a change from one Plan to another. However, it allows identifying the unique combination of Payer (insurer), Plan (determining healthcare benefits and limits) and Person. Typically, depending on healthcare utilization, a Person may have one or many subsequent Plans during coverage by a single Payer.
- Payer or Plan information is not normalized or recorded as part of the Standard Vocabularies. Instead, they are referred to only by their source_value.
- Typically, family members are covered under the same Plan as the Person. In those cases, the payer_source_value, plan_source_value and family_source_value are identical.
VISIT_COST table
As of version 5.0.1 (5-Apr-2016), this table is no longer part of the OMOP CDM. It is replaced by the COST table.
For prior definition, see below.
The VISIT_COST table captures the cost of a Visit of a Person not itemized to specific procedures, drugs, or devices used during the Visit.
| Field | Required | Type | Description |
|---|---|---|---|
| visit_cost_id | Yes | integer | A unique identifier for each procedure cost record. |
| visit_occurrence_id | Yes | integer | A foreign key identifier to the procedure record for which cost data are recorded. |
| currency_concept_id | No | integer | A concept representing the 3-letter code used to delineate international currencies, such as USD for US Dollar. |
| paid_copay | No | float | The amount paid by the Person as a fixed contribution to the expenses. Copay does not contribute to the out_of_pocket expenses. |
| paid_coinsurance | No | float | The amount paid by the Person as a joint assumption of risk. Typically, this is a percentage of the expenses defined by the Health benefit Plan after the person's deductible is exceeded. |
| paid_toward_ deductible | No | float | The amount paid by the Person that is counted toward the deductible defined by the health benefit Plan. |
| paid_by_payer | No | float | The amount paid by the Payer (insurer). If there is more than one Payer, several VISIT_COST records indicate that fact. |
| paid_by_coordination_benefits | No | float | The amount paid by a secondary Payer through the coordination of benefits. |
| total_out_of_pocket | No | float | The total amount paid by the Person as a share of the expenses, excluding the copay. |
| total_paid | No | float | The total amount paid for the expenses of the procedure. |
| payer_plan_period_id | No | integer | A foreign key to the PAYER_PLAN_PERIOD table, where the details of the Payer, Plan and Family are stored. |
Conventions
- The cost of the Visit may contain just board and food, but could also include the entire cost of everything that was happening to the patient during the Visit.
- All other conventions apply as in the PROCEDURE_COST table.
PROCEDURE_COST table
As of version 5.0.1 (5-Apr-2016), this table is no longer part of the OMOP CDM. It is replaced by the COST table.
For prior definition, see below.
The PROCEDURE_COST table captures the cost of a Procedure performed on a Person. The information about the cost is only derived from the amounts paid for the Procedure. This is in contrast to the Drug Cost data which also contain information about true amount charged by the distributor. In addition, Revenue codes are captured.
| Field | Required | Type | Description |
|---|---|---|---|
| procedure_cost_id | Yes | integer | A unique identifier for each procedure cost record. |
| procedure_occurrence_id | Yes | integer | A foreign key identifier to the procedure record for which cost data are recorded. |
| currency_concept_id | No | integer | A concept representing the 3-letter code used to delineate international currencies, such as USD for US Dollar. |
| paid_copay | No | float | The amount paid by the Person as a fixed contribution to the expenses. Copay does not contribute to the out_of_pocket expenses. |
| paid_coinsurance | No | float | The amount paid by the Person as a joint assumption of risk. Typically, this is a percentage of the expenses defined by the health benefit Plan after the Person's deductible is exceeded. |
| paid_toward_deductible | No | float | The amount paid by the Person that is counted toward the deductible defined by the health benefit Plan. |
| paid_by_payer | No | float | The amount paid by the Payer. If there is more than one Payer, several PROCEDURE_COST records indicate that fact. |
| paid_by_coordination_benefits | No | float | The amount paid by a secondary Payer through the coordination of benefits. |
| total_out_of_pocket | No | float | The total amount paid by the Person as a share of the expenses |
| total_paid | No | float | The total amount paid for the expenses of the Procedure. |
| revenue_code_concept_id | No | integer | A foreign key referring to a Standard Concept ID in the Standardized Vocabularies for Revenue codes. |
| payer_plan_period_id | No | integer | A foreign key to the PAYER_PLAN_PERIOD table, where the details of the payer, plan and family are stored. |
| revenue_code_source_value | No | varchar(50) | The source code for the Revenue code as it appears in the source data, stored here for reference. |
Conventions
- Each Procedure Occurrence may have any number of corresponding records in the PROCEDURE_COST table, but often it is none (cost data not captured) or one (one payment per Procedure). They are linked directly through the Procedure Occurrence ID field.
- If Procedures payments are bundled and the cost of such a bundle might be represented in only one of the component Procedures. The FACT_RELATIONSHIP table contains the relationship between the charged Procedure and the Procedure belonging to the bundle.
- The amounts paid are:
- Copay – a fixed amount to be paid by the Person
- Coinsurance – a relative amount of the total paid by the Person
- Deductible – an amount of money paid by the Person before the Payer starts contributing
- Primary Payer – the amount the primary Payer pays towards the total
- Coordination of Benefits – the amount a secondary Payer or Family Plan pays towards the total
- Out of Pocket = Copay + Coinsurance + Deductible
- Total – the total amount paid for the Procedure
- The amounts in various payment components should equal the total, so Copay + Coinsurance + Deductible + Primary Payer + COB = Total Paid. In reality, this is not always reflected in the source data. It is up to the ETL to determine how to deal with quality problems in the data.
- The revenue_code_concept_id determines what service within a provider is charging for the service
DRUG_COST table
As of version 5.0.1 (5-Apr-2016), this table is no longer part of the OMOP CDM. It is replaced by the COST table.
For prior definition, see below.
The DRUG_COST table captures records containing the cost of a Drug Exposure. The information about the cost is defined by the amount of money paid by the Person and Payer for the Drug, as well as the charged cost of the Drug. In addition, a reference to the health plan information in the PAYER_PLAN_PERIOD table is stored in the record that is responsible for the determination of the cost as well as some of the Payments.
| Field | Required | Type | Description |
|---|---|---|---|
| drug_cost_id | Yes | integer | A unique identifier for each DRUG_COST record. |
| drug_exposure_id | Yes | integer | A foreign key identifier to the Drug record for which cost data are recorded. |
| currency_concept_id | No | integer | A concept representing the 3-letter code used to delineate international currencies, such as USD for US Dollar. |
| paid_copay | No | float | The amount paid by the Person as a fixed contribution to the expenses. Copay does not contribute to the out of pocket expenses. |
| paid_coinsurance | No | float | The amount paid by the Person as a joint assumption of risk. Typically, this is a percentage of the expenses defined by the Payer Plan after the Person's deductible is exceeded. |
| paid_toward_deductible | No | float | The amount paid by the Person that is counted toward the deductible defined by the Payer Plan. |
| paid_by_payer | No | float | The amount paid by the Payer. If there is more than one Payer, several DRUG_COST records indicate that fact. |
| paid_by_coordination_benefits | No | float | The amount paid by a secondary Payer through the coordination of benefits. |
| total_out_of_pocket | No | float | The total amount paid by the Person as a share of the expenses. |
| total_paid | No | float | The total amount paid for the expenses of drug exposure. |
| ingredient_cost | No | float | The portion of the drug expenses due to the cost charged by the manufacturer for the drug, typically a percentage of the Average Wholesale Price. |
| dispensing_fee | No | float | The portion of the drug expenses due to the dispensing fee charged by the pharmacy, typically a fixed amount. |
| average_wholesale_price | No | float | List price of a Drug set by the manufacturer. |
| payer_plan_period_id | No | integer | A foreign key to the PAYER_PLAN_PERIOD table, where the details of the Payer, Plan and Family are stored. |
Conventions
- Each Drug Exposure may have any number of corresponding records in the DRUG_COST table, but usually it is none (no cost data recorded) or one. They are linked directly through the drug_exposure_id field.
- The amounts paid are:
- Copay – a fixed amount to be paid by the Person
- Coinsurance – a relative amount of the total paid by the Person
- Deductible – an amount of money paid by the Person before the Payer starts contributing
- Primary Payer – the amount the primary Payer pays towards the total
- Coordination of Benefits – the amount a secondary Payer or Family Plan pays towards the total
- Out of Pocket = Copay + Coinsurance + Deductible
- Total – the total amount paid for the Drug Exposure
- Drug costs are:
- Ingredient Cost – the amount charged by the wholesale distributor or manufacturer
- Dispensing Fee – the amount charged by the pharmacy
- Sales Tax. This is usually very small and typically not provided by most source data, and therefore not included in the CDM
- The amount paid should equal the cost, so Copay + Coinsurance + Deductible + Primary Payer + Coordination of Benefits = Total Paid = Ingredient Cost + Dispensing Fee. In reality, this is not always reflected in the source data. It is up to the ETL to determine how to deal with quality problems in the data.
- The Average Wholesale Price is the list price of the drug, but not the price actually charged or paid.
DEVICE_COST table
As of version 5.0.1 (5-Apr-2016), this table is no longer part of the OMOP CDM. It is replaced by the COST table.
For prior definition, see below.
The DEVICE_COST table captures the cost of a medical Device or supply used on a Person. The information about the cost is only derived from the amounts paid for the device.
| Field | Required | Type | Description |
|---|---|---|---|
| device_cost_id | Yes | integer | A unique identifier for each DEVICE_COST record. |
| device_exposure_ id | Yes | integer | A foreign key identifier to the DEVICE_EXPOSURE record for which cost data are recorded. |
| currency_concept_id | No | integer | A concept representing the 3-letter code used to delineate international currencies, such as USD for US Dollar. |
| paid_copay | No | float | The amount paid by the Person as a fixed contribution to the expenses. Copay does not contribute to the out_of_pocket expenses. |
| paid_coinsurance | No | float | The amount paid by the Person as a joint assumption of risk. Typically, this is a percentage of the expenses defined by the Payer Plan after the person's deductible is exceeded. |
| paid_toward_ deductible | No | float | The amount paid by the Person that is counted toward the deductible defined by the Payer Plan. |
| paid_by_payer | No | float | The amount paid by the Payer. If there is more than one payer, several procedure_cost records indicate that fact. |
| paid_by_coordination_benefits | No | float | The amount paid by a secondary payer through the coordination of benefits. |
| total_out_of_pocket | No | float | The total amount paid by the Person as a share of the expenses, excluding the copay. |
| total_paid | No | float | The total amount paid for the expenses of the procedure. |
| payer_plan_period_id | No | integer | A foreign key to the payer_plan_period table, where the details of the payer, plan and family are stored. |
Conventions
- If the Device is derived from a Procedure record, all conventions apply to the field’s equivalent to the procedure_cost (see above).
Standardized Derived Elements
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
These tables contain information about the clinical events of a patient that are not obtained directly from the raw source data, but from other tables of the CDM.
Below provides an entity-relationship diagram highlighting the tables within the Standardized Derived Elements portion of the OMOP Common Data Model:
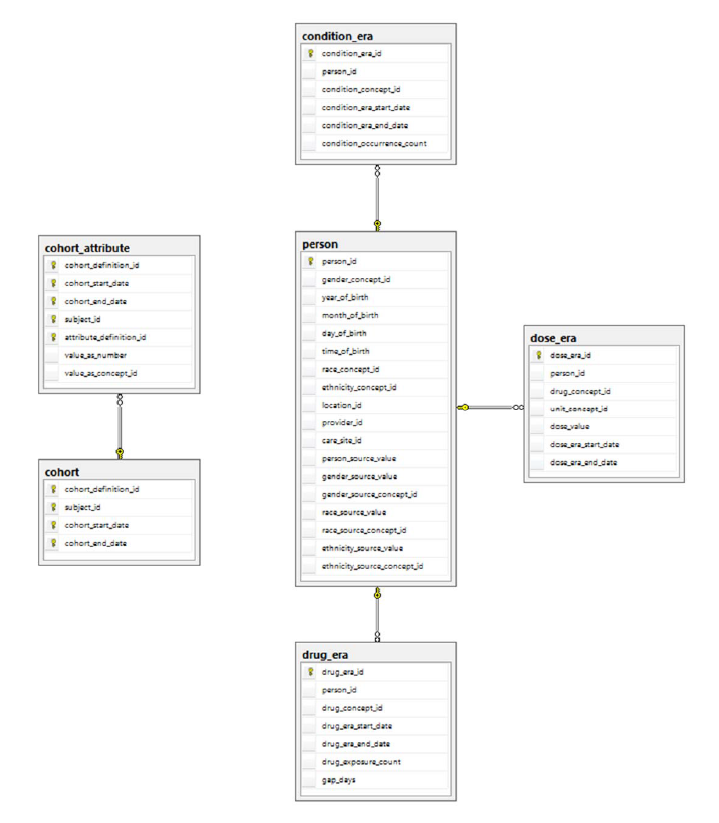
COHORT table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The COHORT table contains records of subjects that satisfy a given set of criteria for a duration of time. The definition of the cohort is contained within the COHORT_DEFINITION table. Cohorts can be constructed of patients (Persons), Providers or Visits.
| Field | Required | Type | Description |
|---|---|---|---|
| cohort_definition_id | Yes | integer | A foreign key to a record in the COHORT_DEFINITION table containing relevant Cohort Definition information. |
| subject_id | Yes | integer | A foreign key to the subject in the cohort. These could be referring to records in the PERSON, PROVIDER, VISIT_OCCURRENCE table. |
| cohort_start_date | Yes | date | The date when the Cohort Definition criteria for the Person, Provider or Visit first match. |
| cohort_end_date | Yes | date | The date when the Cohort Definition criteria for the Person, Provider or Visit no longer match or the Cohort membership was terminated. |
Conventions
- The core of a Cohort is the unifying definition or feature of the Cohort. This is captured in the cohort_definition_id. For example, Cohorts can include patients diagnosed with a specific condition, patients exposed to a particular drug, or Providers who have performed a specific Procedure.
- Cohort records must have a Start Date
- Cohort records must have an End Date, but may be set to Start Date or could have applied a censored date using the Observation Period Start Date.
- Cohort records must contain a Subject Id, which can refer to the Person, Provider, or Visit record. The Cohort Definition will define the type of subject through the subject concept id.
COHORT_ATTRIBUTE table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
The COHORT_ATTRIBUTE table contains attributes associated with each subject within a cohort, as defined by a given set of criteria for a duration of time. The definition of the Cohort Attribute is contained in the ATTRIBUTE_DEFINITION table.
| Field | Required | Type | Description |
|---|---|---|---|
| cohort_definition_id | Yes | integer | A foreign key to a record in the COHORT_DEFINITION table containing relevant Cohort Definition information. |
| subject_id | Yes | integer | A foreign key to the subject in the Cohort. These could be referring to records in the PERSON, PROVIDER, VISIT_OCCURRENCE table. |
| cohort_start_date | Yes | date | The date when the Cohort Definition criteria for the Person, Provider or Visit first match. |
| cohort_end_date | Yes | date | The date when the Cohort Definition criteria for the Person, Provider or Visit no longer match or the Cohort membership was terminated. |
| attribute_definition_id | Yes | integer | A foreign key to a record in the ATTRIBUTE_DEFINITION table containing relevant Attribute Definition information. |
| value_as_number | No | float | The attribute result stored as a number. This is applicable to attributes where the result is expressed as a numeric value. |
| value_as_concept_id | No | integer | The attribute result stored as a Concept ID. This is applicable to attributes where the result is expressed as a categorical value. |
Conventions
- Each record in the COHORT_ATTRIBUTE table is linked to a specific record in the COHORT table, identified by matching cohort_definition_id, subject_id, cohort_start_date and cohort_end_date fields.
- It adds to the Cohort records calculated co-variates (for example age, BMI) or composite scales (for example Charleson index).
- The unifying definition or feature of the Cohort Attribute is captured in the attribute_definition_id referring to a record in the ATTRIBUTE_DEFINITION table.
- The actual result or value of the Cohort Attribute (co-variate, index value) is captured in the value_as_number (if the value is numberic) or the value_as_concept_id (if the value is a concept) fields.
DRUG_ERA table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
A Drug Era is defined as a span of time when the Person is assumed to be exposed to a particular active ingredient. A Drug Era is not the same as a Drug Exposure: Exposures are individual records corresponding to the source when Drug was delivered to the Person, while successive periods of Drug Exposures are combined under certain rules to produce continuous Drug Eras.
| Field | Required | Type | Description |
|---|---|---|---|
| drug_era_id | Yes | integer | A unique identifier for each Drug Era. |
| person_id | Yes | integer | A foreign key identifier to the Person who is subjected to the Drug during the fDrug Era. The demographic details of that Person are stored in the PERSON table. |
| drug_concept_id | Yes | integer | A foreign key that refers to a Standard Concept identifier in the Standardized Vocabularies for the Ingredient Concept. |
| drug_era_start_date | Yes | date | The start date for the Drug Era constructed from the individual instances of Drug Exposures. It is the start date of the very first chronologically recorded instance of conutilization of a Drug. |
| drug_era_end_date | Yes | date | The end date for the drug era constructed from the individual instance of drug exposures. It is the end date of the final continuously recorded instance of utilization of a drug. |
| drug_exposure_count | No | integer | The number of individual Drug Exposure occurrences used to construct the Drug Era. |
| gap_days | No | integer | The number of days that are not covered by DRUG_EXPOSURE records that were used to make up the era record. |
Conventions
- Drug Eras are derived from records in the DRUG_EXPOSURE table using a standardized algorithm.
- Each Drug Era corresponds to one or many Drug Exposures that form a continuous interval and contain the same Drug Ingredient (active compound).
- The drug_concept_id field only contains Concepts that have the concept_class 'Ingredient'. The Ingredient is derived from the Drug Concepts in the DRUG_EXPOSURE table that are aggregated into the Drug Era record.
- The Drug Era Start Date is the start date of the first Drug Exposure.
- The Drug Era End Date is the end date of the last Drug Exposure. The End Date of each Drug Exposure is either taken from the field drug_exposure_end_date or, as it is typically not available, inferred using the following rules:
- For pharmacy prescription data, the date when the drug was dispensed plus the number of days of supply are used to extrapolate the End Date for the Drug Exposure. Depending on the country-specific healthcare system, this supply information is either explicitly provided in the day_supply field or inferred from package size or similar information.
- For Procedure Drugs, usually the drug is administered on a single date (i.e., the administration date).
- A standard Persistence Window of 30 days (gap, slack) is permitted between two subsequent such extrapolated DRUG_EXPOSURE records to be considered to be merged into a single Drug Era.
- The Gap Days determine how many total drug-free days are observed between all Drug Exposure events that contribute to a DRUG_ERA record. It is assumed that the drugs are “not stockpiled” by the patient, i.e. that if a new drug prescription or refill is observed (a new DRUG_EXPOSURE record is written), the remaining supply from the previous events is abandoned.
- The difference between Persistence Window and Gap Days is that the former is the maximum drug-free time allowed between two subsequent DRUG_EXPOSURE records, while the latter is the sum of actual drug-free days for the given Drug Era under the above assumption of non-stockpiling.
- The choice of a standard Persistence Window of 30 and the non-stockpiling assumption is arbitrary, but has been shown to deliver good results in drug-outcome estimation. Other problems, such as estimation of drug compliance, my require a different or drug-dependent Persistence Window/stockpiling assumption. Researchers are encouraged to consider creating their own Drug Eras with different parameters as Cohorts and store them in the COHORT table.
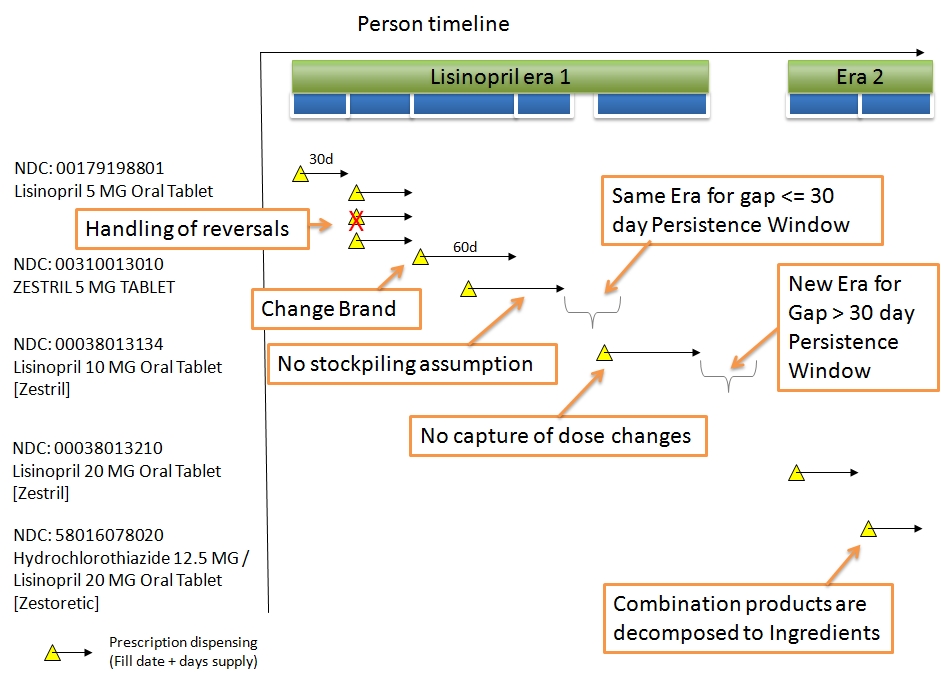
DOSE_ERA table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
A Dose Era is defined as a span of time when the Person is assumed to be exposed to a constant dose of a specific active ingredient.
| Field | Required | Type | Description |
|---|---|---|---|
| dose_era_id | Yes | integer | A unique identifier for each Dose Era. |
| person_id | Yes | integer | A foreign key identifier to the Person who is subjected to the drug during the drug era. The demographic details of that Person are stored in the PERSON table. |
| drug_concept_id | Yes | integer | A foreign key that refers to a Standard Concept identifier in the Standardized Vocabularies for the active Ingredient Concept. |
| unit_concept_id | Yes | integer | A foreign key that refers to a Standard Concept identifier in the Standardized Vocabularies for the unit concept. |
| dose_value | Yes | float | The numeric value of the dose. |
| dose_era_start_date | Yes | date | The start date for the drug era constructed from the individual instances of drug exposures. It is the start date of the very first chronologically recorded instance of utilization of a drug. |
| dose_era_end_date | Yes | date | The end date for the drug era constructed from the individual instance of drug exposures. It is the end date of the final continuously recorded instance of utilization of a drug. |
Conventions
- Dose Eras will be derived from records in the DRUG_EXPOSURE table and the Dose information from the DRUG_STRENGTH table using a standardized algorithm.
- Each Dose Era corresponds to one or many Drug Exposures that form a continuous interval and contain the same Drug Ingredient (active compound) at the same effective daily dose.
- Dose Form information is not taken into account. So, if the patient changes between different formuations, or different manufacturers with the same formulation, the Dose Era is still spanning the entire time of exposure to the Ingredient.
- The daily dose is calculated for each DRUG_EXPOSURE record by calculating the total dose of the record and dividing by the duration.
- The total dose of a DRUG_EXPOSURE record is calculated with the help of the DRUG_STRENGTH table containing the dosage information for each drug as following:
| 1 | Tablets and other fixed amount formulations |
|---|---|
| Example: Acetaminophen (Paracetamol) 500 mg, 20 tablets. | |
| DRUG_STRENGTH | The denominator_unit is empty |
| DRUG_EXPOSURE | The quantity refers to number of pieces, e.g. tablets |
| In the example: 20 | |
Ingredient dose= | quantity x amount_value [amount_unit_concept_id] |
Acetaminophen dose = 20 x 500mg = 10,000mg |
|
| 2 | Puffs of an inhaler |
| Note: There is no difference to use case 1 besides that the DRUG_STRENGTH table may put {actuat} in the denominator unit. In this case the strength is provided in the numerator. | |
| DRUG_STRENGTH | The denominator_unit is {actuat} |
| DRUG_EXPOSURE | The quantity refers to the number of pieces, e.g. puffs |
Ingredient dose= | quantity x numerator_value [numerator_unit_concept_id] |
| 3 | Quantified Drugs which are formulated as a concentration |
| Example: The Clinical Drug is Acetaminophen 250 mg/mL in a 5mL oral suspension. The Quantified Clinical Drug would have 1250 mg / 5 ml in the DRUG_STRENGTH table. Two suspensions are dispensed. | |
| DRUG_STRENGTH | The denominator_unit is either mg or mL. The denominator_value might be different from 1. |
| DRUG_EXPOSURE | The quantity refers to a fraction or, multiple of the pack. |
| Example: 2 | |
Ingredient dose= | quantity x numerator_value [numerator_unit_concept_id] |
Acetaminophen dose = 2 x 1250mg = 2500mg |
|
| 4 | Drugs with the total amount provided in quantity, e.g. chemotherapeutics |
| Example: 42799258 “Benzyl Alcohol 0.1 ML/ML / Pramoxine hydrochloride 0.01 MG/MG Topical Gel” dispensed in a 1.25oz pack. | |
| DRUG_STRENGTH | The denominator_unit is either mg or mL. |
| Example: Benzyl Alcohol in mL and Pramoxine hydrochloride in mg | |
| DRUG_EXPOSURE | The quantity refers to mL or g. |
| Example: 1.25 x 30 (conversion factor oz → mL) = 37 | |
Ingredient dose= | quantity x numerator_value [numerator_unit_concept_id] |
Benzyl Alcohol dose = 37 x 0.1mL = 3.7mL |
|
Pramoxine hydrochloride dose = 37 x 0.01mg x 1000 = 370mg |
|
| Note: The analytical side should check the denominator in the DRUG_STRENGTH table. As mg is used for the second ingredient the factor 1000 will be applied to convert between g and mg. | |
| 5 | Compounded drugs |
| Example: Ibuprofen 20%/Piroxicam 1% Cream, 30ml in 5ml tubes. | |
| DRUG_STRENGTH | We need entries for the ingredients of Ibuprofen and Piroxicam, probably with an amount_value of 1 and a unit of mg. |
| DRUG_EXPOSURE | The quantity refers to the total amount of the compound. Use one record in the DRUG_EXPOSURE table for each compound. |
| Example: 20% Ibuprofen of 30ml = 6mL, 1% Piroxicam of 30ml = 0.3mL | |
Ingredient dose= | Depends on the drugs involved: One of the use cases above. |
Ibuprofen dose = 6 x 1mg x 1000 = 6000mg |
|
Piroxicam dose = 0.3 x 1mg x 1000 = 300mg |
|
| Note: The analytical side determines that the denominator for both ingredients in the DRUG_STRENGTH table is mg and applies the factor 1000 to convert between mL/g and mg. | |
| 6 | Drugs with the active ingredient released over time, e.g. patches |
| Example: Ethinyl Estradiol 0.000833 MG/HR / norelgestromin 0.00625 MG/HR Weekly Transdermal Patch | |
| DRUG_STRENGTH | The denominator units refer to hour. |
| Example: Ethinyl Estradiol 0.000833 mg/h / norelgestromin 0.00625 mg/h | |
| DRUG_EXPOSURE | The quantity refers to the number of pieces. |
| Example: 1 patch | |
Ingredient rate= | numerator_value [numerator_unit_concept_id] |
Ethinyl Estradiol rate = 0.000833 mg/h |
|
norelgestromin rate 0.00625 mg/h |
|
| Note: This can be converted to a daily dosage by multiplying it with 24. (Assuming 1 patch at a time for at least 24 hours) |
CONDITION_ERA table
THIS IS OUTDATED. All documentation is now on the github wiki. Please refer there or to the CDM working group for more information
A Condition Era is defined as a span of time when the Person is assumed to have a given condition. Similar to Drug Eras, Condition Eras are chronological periods of Condition Occurrence. Combining individual Condition Occurrences into a single Condition Era serves two purposes:
- It allows aggregation of chronic conditions that require frequent ongoing care, instead of treating each Condition Occurrence as an independent event.
- It allows aggregation of multiple, closely timed doctor visits for the same Condition to avoid double-counting the Condition Occurrences.
For example, consider a Person who visits her Primary Care Physician (PCP) and who is referred to a specialist. At a later time, the Person visits the specialist, who confirms the PCP’s original diagnosis and provides the appropriate treatment to resolve the condition. These two independent doctor visits should be aggregated into one Condition Era.
| Field | Required | Type | Description |
|---|---|---|---|
| condition_era_id | Yes | integer | A unique identifier for each Condition Era. |
| person_id | Yes | integer | A foreign key identifier to the Person who is experiencing the Condition during the Condition Era. The demographic details of that Person are stored in the PERSON table. |
| condition_concept_id | Yes | integer | A foreign key that refers to a standard Condition Concept identifier in the Standardized Vocabularies. |
| condition_era_start_date | Yes | date | The start date for the Condition Era constructed from the individual instances of Condition Occurrences. It is the start date of the very first chronologically recorded instance of the condition. |
| condition_era_end_date | Yes | date | The end date for the Condition Era constructed from the individual instances of Condition Occurrences. It is the end date of the final continuously recorded instance of the Condition. |
| condition_occurrence_count | No | integer | The number of individual Condition Occurrences used to construct the condition era. |
Conventions
- Condition Era records will be derived from the records in the CONDITION_OCCURRENCE table using a standardized algorithm.
- Each Condition Era corresponds to one or many Condition Occurrence records that form a continuous interval.
The condition_concept_id field contains Concepts that are identical to those of the CONDITION_OCCURRENCE table records that make up the Condition Era. In contrast to Drug Eras, Condition Eras are not aggregated to contain Conditions of different hierarchical layers. The Condition Era Start Date is the start date of the first Condition Occurrence. The Condition Era End Date is the end date of the last Condition Occurrence.
- Condition Eras are built with a Persistence Window of 30 days, meaning, if no occurence of the same condition_concept_id happens within 30 days of any one occurrence, it will be considered the condition_era_end_date.