- Who We Are
- Updates & News
- Standards
- Software Tools
- Network Studies
- Community Forums
- Education
- New To OHDSI?
- Community Calls
- Past Events
- Workgroups
- Tutorials
- 2025 ‘Our Journey’ Annual Report
- Current Events
- Support & Sponsorship
- 2025 Global Symposium
- 2026 Europe Symposium
- 2026 Global Symposium
- Github
- YouTube
- X/Twitter
- Newsletters

2025 OHDSI Global Symposium
Oct. 7-9 • New Brunswick, N.J. • Hyatt Regency Hotel
The 11th annual OHDSI Global Symposium emphasized building trust in science through global collaboration in network studies, drawing more than 400 collaborators from six continents. Across three days of research presentations, discussions, and community networking, participants advanced OHDSI’s mission of improving health by generating the evidence that supports better decisions and better care worldwide.
This page will host all OHDSI2025 materials, including video presentations (when available) from the main conference and tutorials, slide decks, posters, demos, and more.

State of the Community
George Hripcsak welcomed the community to the 11th OHDSI Symposium. During this talk, George shared recent highlights within OHDSI, shared some key ways to measure truth in science, and welcomed some of the community’s global leaders to the stage to share more advances within the community.
Plenary: Why network studies are necessary to improve trust in evidence
Plenary: Why network studies are necessary to improve trust in evidence
Presenters: Martijn Schuemie, Johnson & Johnson; Asieh Golozar, Nemesis Health; Cindy Cai, Johns Hopkins University; Patrick Ryan, Johnson & Johnson, Columbia University
Plenary: Reflections on the evolution of pre- and postmarket safety review in CDER over 3 decades
Plenary: Reflections on the evolution of pre- and postmarket safety review in CDER over 3 decades
Presenter: Judy Racoosin, US Food and Drug Administration (retired)
Collaborator Showcase Lightning Talks: Session 1
Bridging Standards: Creating OMOP data via Fast Healthcare Interoperability Resources (FHIR) and Health Information Networks (Stephanie Hong, Johns Hopkins University)
OMOP Waveform Extension: A Schema for Integrating Physiological Signals and Derived Features into the OMOP CDM (Jared Houghtaling, Tufts University)
Improving VSAC to OMOP Mapping Using LLM Assisted Curation (Robert Barrett, Johns Hopkins University)
Evaluating the effectiveness of using Large Language Models for the development of concept sets (Joel Swerdel, Johnson & Johnson)
Validating a Scalable Approach to Data Fitness-for-Use: Database Diagnostics Applied to LEGEND-T2DM (Clair Blacketer, Johnson & Johnson)
Moderator: Harry Reyes Nieva (Columbia University)
Collaborator Showcase Lightning Talks: Session 2
Causal Inference with Multi-Modal Foundation Models: A Case Study of Anti-VEGF Injections in Diabetic Macular Edema (Linying Zhang, Washington University in St. Louis)
LATTE: A One-shot Lossless Algorithm for Federated Target Trial Emulation with Application to Alzheimer’s Disease and Related Dementia Drug Repurposing Using Decentralized Data (Lu Li, University of Pennsylvania)
From Data Quality to Clinical Quality – Episodes as Enablers for Next Generation Dashboarding (Georgina Kennedy, Ingham Institute for Applied Medical Research)
Heterogeneity of Treatment Effects Across Nine Glucose-Lowering Drug Classes in Type 2 Diabetes: Extension of the LEGEND-T2DM Network Study (Hsin Yi “Cindy” Chen, Columbia University)
DARWIN EU® – A multi-national network cohort and self-controlled case series study of the effect of doxycycline versus active comparators on the risk of suicidality in individuals with acne (Katia Verhamme, Erasmus MC)
Moderator: Ben Martin (Johns Hopkins University)
Closing Session / Titans Presentation
Patrick Ryan led the closing session by presenting the 2025 Titan Awards (see below) before voicing the critical need for greater global collaboration. He introduced an activity to end the day that included blown-up globes, post-it notes and 15 minutes of chaos in the Hyatt Regency Ballroom. Congratulations to our 2025 Titan Award winners, as announced during the Global Symposium!
Data Standards: Polina Talapova
Methodological Research: Joel Swerdel
Open-Source Development: Aniek Markus, Maarten van Kessel, Jared Houghtaling
Clinical Applications: DARWIN EU Team
Community Collaboration: Liesbet Peeters, Ilse Vermeulen, Lotte Geys
Community Support: Agnes Kiragga
Community Leadership: Greg Klebanov
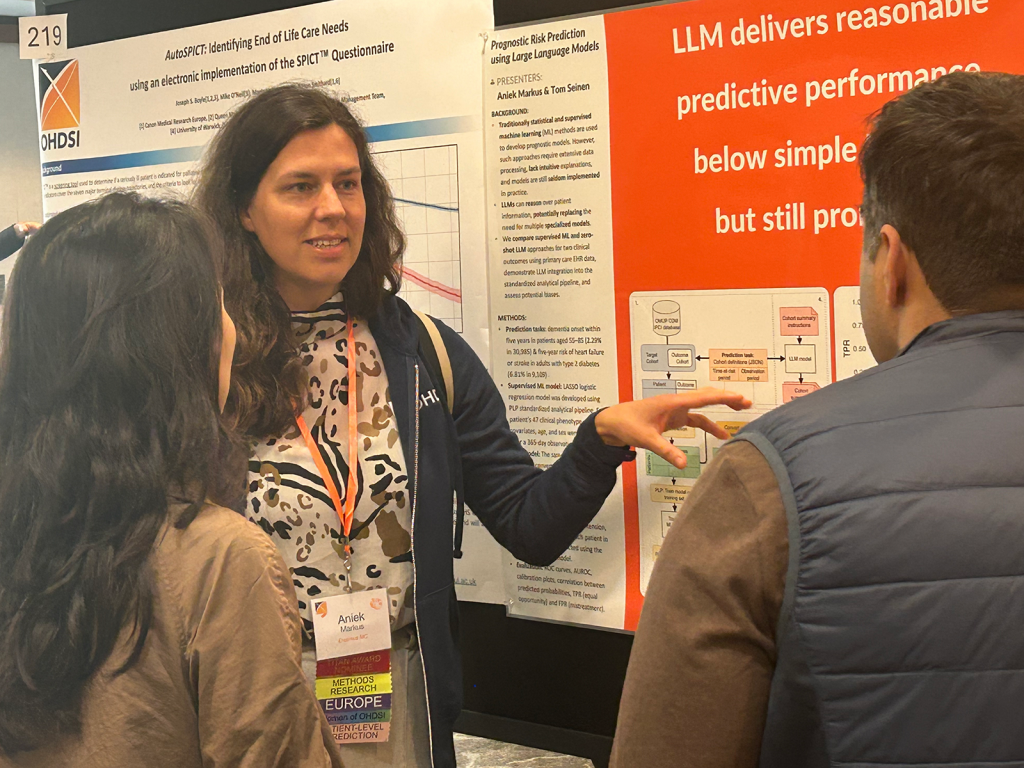
Collaborator Showcase Posters, Demos & Lightning Talks
 There were more than 130 accepted submissions for the 2025 Collaborator Showcase, including posters, software demos and the lightning talks you can find above. This research was both developed and peer-reviewed within the community, and it focuses on several pillars of OHDSI: data standards, methodological research, open-source development, clinical applications, and building community.
There were more than 130 accepted submissions for the 2025 Collaborator Showcase, including posters, software demos and the lightning talks you can find above. This research was both developed and peer-reviewed within the community, and it focuses on several pillars of OHDSI: data standards, methodological research, open-source development, clinical applications, and building community.
Please visit the link below to visit the posters, brief reports and other supplementary materials for our showcase submissions. Each submission will be profiled in the #OHDSISocialShowcase, so please make sure you follow OHDSI on LinkedIn, Twitter/X, Instagram, and Bluesky.
Tutorials
An Introduction to the Journey from Data to Evidence Using OHDSI
Faculty: Erica Voss, Yong Chen, Katy Sadowski, Nicole Pratt, Roger Carlson, Chongliang (Jason) Luo
Developing and Evaluating Your Extract, Transform, Load (ETL) Process to the OMOP Common Data Model
Faculty: Clair Blacketer, Karthik Natarajan, Evanette Burrows, Max Adulyanuksol, Maxim Moinat
Using the OHDSI Standardized Vocabularies for Research
Faculty: Anna Ostropolets, Vlad Korsik, Polina Talapova, Masha Khitrun
Clinical Characterization Applications to Generate Reliable Real-World Evidence
Faculty: Patrick Ryan, Aniek Markus, Hsin Yi “Cindy” Chen, Azza Shoaibi
Population-Level Effect Estimation Applications to Generate Reliable Real-World Evidence
Faculty: George Hripcsak, Martijn Schuemie, Linying Zhang, Tara Anand
Patient-Level Prediction Applications to Generate Reliable Real-World Evidence
Faculty: Jenna Reps, Egill Fridgersson, Ross Williams
Thank You To Our Global Symposium Sponsors

